Ampicillin and Sulbactam
AMPICILLIN AND SULBACTAM FOR INJECTION, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMPICILLIN AND SULBACTAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- AMPICILLIN AND SULBACTAM INDICATIONS AND USAGE
- AMPICILLIN AND SULBACTAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMPICILLIN AND SULBACTAM ADVERSE REACTIONS
- OVERDOSAGE
- CLINICAL STUDIES
- AMPICILLIN AND SULBACTAM DOSAGE AND ADMINISTRATION
- COMPATIBILITY, RECONSTITUTION AND STABILITY
- DIRECTIONS FOR USE
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL - 1.5 gram
- PRINCIPAL DISPLAY PANEL - 3 gram
FULL PRESCRIBING INFORMATION
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin and Sulbactam for Injection, USP and other antibacterial drugs, Ampicillin and Sulbactam for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Ampicillin and Sulbactam for Injection, USP is a sterile injectable antibacterial combination consisting of the semisynthetic antibiotic ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration.
Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylate and has a molecular weight of 371.41. Its chemical formula is C16H18N3NaO4S. The structural formula is:
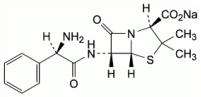
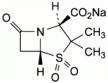
Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactamsodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0]heptane-2-carboxylate 4,4-dioxide.
Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22. The structural formula is:
Ampicillin and Sulbactam for Injection parenteral combination is available as a white to off-white dry powder for reconstitution. Ampicillin and Sulbactam for Injection dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. The pH of the solutions is between 8 and 10.
Dilute solutions (up to 30 mg ampicillin and 15 mg sulbactam per mL) are essentially colorless to pale yellow. The pH of dilute solutions remains the same. 1.5 g of Ampicillin and Sulbactam for Injection (1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt) parenteral contains approximately 115 mg (5 mEq) of sodium.
3 g of Ampicillin and Sulbactam for Injection (2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt) parenteral contains approximately 230 mg (10 mEq) of sodium.
CLINICAL PHARMACOLOGY
General
Immediately after completion of a 15-minute intravenous infusion of Ampicillin and Sulbactam for Injection, peak serum concentrations of ampicillin and sulbactam are attained. Ampicillin serum levels are similar to those produced by the administration of equivalent amounts of ampicillin alone. Peak ampicillin serum levels ranging from 109 to 150 mcg/mL are attained after administration of 2000 mg of ampicillin plus 1000 mg sulbactam and 40 to 71 mcg/mL after administration of 1000 mg ampicillin plus 500 mg sulbactam. The corresponding mean peak serum levels for sulbactam range from 48 to 88 mcg/mL and 21 to 40 mcg/mL, respectively. After an intramuscular injection of 1000 mg ampicillin plus 500 mg sulbactam, peak ampicillin serum levels ranging from 8 to 37 mcg/mL and peak sulbactam serum levels ranging from 6 to 24 mcg/mL are attained. The mean serum half-life of both drugs is approximately 1 hour in healthy volunteers.
Approximately 75 to 85% of both ampicillin and sulbactam are excreted unchanged in the urine during the first 8 hours after administration of Ampicillin and Sulbactam for Injection to individuals with normal renal function. Somewhat higher and more prolonged serum levels of ampicillin and sulbactam can be achieved with the concurrent administration of probenecid.
In patients with impaired renal function the elimination kinetics of ampicillin and sulbactam are similarly affected, hence the ratio of one to the other will remain constant whatever the renal function. The dose of Ampicillin and Sulbactam for Injection in such patients should be administered less frequently in accordance with the usual practice for ampicillin (see DOSAGE and ADMINISTRATION section).
Ampicillin has been found to be approximately 28% reversibly bound to human serum protein and sulbactam approximately 38% reversibly bound.
The following average levels of ampicillin and sulbactam were measured in the tissues and fluids listed:
| Fluid or Tissue | Dose (grams) Ampicillin/ Sulbactam |
Concentration (mcg/mL or mcg/g) Ampicillin/ Sulbactam |
| Peritoneal Fluid | 0.5/0.5 IV | 7/14 |
| Blister Fluid (Cantharides) |
0.5/0.5 IV | 8/20 |
| Tissue Fluid | 1/0.5 IV | 8/4 |
| Intestinal Mucosa | 0.5/0.5 IV | 11/18 |
| Appendix | 2/1 IV | 3/40 |
Penetration of both ampicillin and sulbactam into cerebrospinal fluid in the presence of inflamed meninges has been demonstrated after IV administration of Ampicillin and Sulbactam.
The pharmacokinetics of ampicillin and sulbactam in pediatric patients receiving Ampicillin and Sulbactam are similar to those observed in adults. Immediately after a 15-minute infusion of 50 to 75 mg Ampicillin and Sulbactam for Injection /kg body weight, peak serum and plasma concentrations of 82 to 446 mcg ampicillin/mL and 44 to 203 mcg sulbactam/mL were obtained. Mean half-life values were approximately 1 hour.
MICROBIOLOGY
Ampicillin is similar to benzyl penicillin in its bactericidal action against susceptible organisms during the stage of active multiplication. It acts through the inhibition of cell wall mucopeptide biosynthesis. Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gram-negative aerobic and anaerobic bacteria. (Ampicillin is, however, degraded by beta-lactamases, and therefore the spectrum of activity does not normally include organisms which produce these enzymes.)
A wide range of beta-lactamases found in microorganisms resistant to penicillins and cephalosporins have been shown in biochemical studies with cell-free bacterial systems to be irreversibly inhibited by sulbactam. Although sulbactam alone possesses little useful antibacterial activity except against the Neisseriaceae, whole organism studies have shown that sulbactam restores ampicillin activity against beta-lactamase producing strains. In particular, sulbactam has good inhibitory activity against the clinically important plasmid mediated beta-lactamases most frequently responsible for transferred drug resistance. Sulbactam has no effect on the activity of ampicillin against ampicillin susceptible strains.
The presence of sulbactam in the Ampicillin and Sulbactam for Injection formulation effectively extends the antibiotic spectrum of ampicillin to include many bacteria normally resistant to it and to other beta-lactam antibiotics. Thus, Ampicillin and Sulbactam possesses the properties of a broad-spectrum antibiotic and a beta-lactamase inhibitor.
While in vitro studies have demonstrated the susceptibility of most strains of the following organisms, clinical efficacy for infections other than those included in the INDICATIONS AND USAGE section has not been documented.
Gram-Positive Bacteria:
Staphylococcus aureus (beta-lactamase and non-beta-lactamase producing),
Staphylococcus epidermidis (beta-lactamase and non-beta-lactamase producing),
Staphylococcus saprophyticus (beta-lactamase and non-beta-lactamase producing),
Streptococcus faecalis † (Enterococcus), Streptococcus pneumoniae † (formerly D. pneumoniae), Streptococcus pyogenes †, Streptococcus viridans †.
Gram-Negative Bacteria:
Hemophilus influenzae (beta-lactamase and non-beta-lactamase producing), Moraxella (Branhamella) catarrhalis (beta-lactamase and non-beta-lactamase producing), Escherichia coli (beta-lactamase and non-beta-lactamase producing), Klebsiella species (all known strains are beta-lactamase producing), Proteus mirabilis (beta-lactamase and non-beta-lactamase producing), Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Morganella morganii, and Neisseria gonorrhoeae (beta-lactamase and non-beta-lactamase producing).
Anaerobes: Clostridium species†, Peptococcus species†, Peptostreptococcus species, Bacteroides species, including B. fragilis.
† These are not beta-lactamase producing strains and, therefore, are susceptible to ampicillin alone.
Susceptibility Testing
Diffusion Technique
For the disk diffusion method of susceptibility testing, a 20 mcg (10 mcg ampicillin + 10 mcg sulbactam) disk should be used. The standardized procedure1,2 requires the use of a standardized inoculum concentration. With this procedure, a report from the laboratory of "Susceptible" indicates that the infecting organism is likely to respond to Ampicillin and Sulbactam for Injection therapy and a report of "Resistant" indicates that the infecting organism is not likely to respond to therapy. An "Intermediate" susceptibility report suggests that the infecting organism would be susceptible to Ampicillin and Sulbactam for Injection if a higher dosage is used or if the infection is confined to tissues or fluids (e.g., urine) in which high antibiotic levels are attained.
Dilution Techniques
Broth, agar, microdilution or equivalent methods may be used to determine the minimal inhibitory concentration (MIC) value for susceptibility of bacterial isolates using standardized methods, inoculums and concentrations of ampicillin/sulbactam1,3,4
The recommended dilution method employs a constant ampicillin/sulbactam ratio of 2:1 in all tubes with increasing concentrations of ampicillin. MIC's are reported in terms of ampicillin concentration in the presence of sulbactam at a constant 2 parts ampicillin to 1 part sulbactam.
TABLE 2
Recommended Ampicillin/Sulbactam, Disk Diffusion and MIC Susceptibility Rangesa,b,c
(Zone diameter in mm)
| Resistant | Intermediate | Susceptible | |
| Gram (-) and Staphylococcus | |||
| Bauer/Kirby | ≤ 11 | 12-14 | ≥ 15 |
| Zone Sizes MIC (mcg of ampicillin/mL) | ≥ 32 | 16 | ≤ 8 |
| Haemophilus influenzae | |||
| Bauer/Kirby | ≤ 19 | -- | ≥ 20 |
| Zone Sizes MIC (mcg of ampicillin/mL) | ≥ 4 | -- | ≤ 2 |
a The non-beta-lactamase producing organisms which are normally susceptible to ampicillin, such as Streptococci, will have similar zone sizes as for ampicillin disks.
b Staphylococci resistant to methicillin, oxacillin, or nafcillin must be considered resistant to ampicillin and sulbactam for injection.
c The quality control cultures should have the following assigned daily ranges for ampicillin/sulbactam (see Table 3):
TABLE 3
|
Disk Diffusion (Zone diameter in mm) |
MIC (mcg/mL ampicillin/ mcg/mL sulbactam) |
||
| E. coli | (ATCC 25922) | 20-25 | 2/1–8/4 |
| S. aureus | (ATCC 25923) | 29–37 | N/A |
| E. coli | (ATCC 35218) | 13–19 | 8/4–32/16 |
| H. influenza | (ATCC 49247) | 14–22 | 2/1-8/4 |
INDICATIONS AND USAGE
Ampicillin and sulbactam for Injection is indicated for the treatment of infections due to susceptible strains of the designated microorganisms in the conditions listed below.
Skin and Skin Structure Infections caused by beta-lactamase producing strains of Staphylococcus aureus, Escherichia coli * , Klebsiella spp.* (including K. pneumoniae * ), Proteus mirabilis * , Bacteroides fragilis * , Enterobacter spp.*, and Acinetobacter calcoaceticus *.
NOTE: For information on use in pediatric patients see PRECAUTIONS, Pediatric Use and CLINICAL STUDIES sections.
Intra-Abdominal Infections caused by beta-lactamase producing strains of Escherichia coli, Klebsiella spp. (including K. pneumoniae * ), Bacteroides spp. (including B. fragilis), and Enterobacter spp.*.
Gynecological Infections caused by beta-lactamase producing strains of Escherichia coli * , and Bacteroides spp.* (including B. fragilis * ).
*Efficacy for this organism in this organ system was studied in fewer than 10 infections.
While Ampicillin and Sulbactam for Injection is indicated only for the conditions listed above, infections caused by ampicillin-susceptible organisms are also amenable to treatment with Ampicillin and Sulbactam for Injection due to its ampicillin content. Therefore, mixed infections caused by ampicillin-susceptible organisms and beta-lactamase producing organisms susceptible to Ampicillin and Sulbactam for Injection should not require the addition of another antibiotic.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify the organisms causing infection and to determine their susceptibility to Ampicillin and Sulbactam for Injection.
Therapy may be instituted prior to obtaining the results from bacteriological and susceptibility studies, when there is reason to believe the infection may involve any of the bbeta-lactamase producing organisms listed above in the indicated organ systems.
Once the results are known, therapy should be adjusted if appropriate.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin and Sulbactam for Injection, USP and other antibacterial drugs, Ampicillin and Sulbactam for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture aand susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology an susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
The use of Ampicillin and Sulbactam for Injection is contraindicated in individuals with a history of hypersensitivity reactions to any of the penicillins.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE APT TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR HYPERSENSITIVITY REACTIONS TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH A PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, AND OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, AMPICILLIN AND SULBACTAM FOR INJECTION SHOULD BE DISCONTINUED AND THE APPROPRIATE THERAPY INSTITUTED.
SERIOUS ANAPHYLACTOID REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin and sulbactam for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
A high percentage of patients with mononucleosis who receive ampicillin develop a skin rash. Thus, ampicillin class antibiotics should not be administered to patients with mmononucleosis. In patients treated with Ampicillin and Sulbactam the possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If ssuperinfections occur (usually involving Pseudomonas or Candida), the drug should be discontinued and/or appropriate therapy instituted.
Prescribing Ampicillin and Sulbactam for Injection, USP in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Patients should be counseled that antibacterial drugs including Ampicillin and Sulbactam for Injection, USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Ampicillin and Sulbactam for Injection, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Ampicillin and Sulbactam for Injection, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Probenecid decreases the renal tubular secretion of ampicillin and sulbactam. Concurrent use of probenecid with Ampicillin and Sulbactam may result in increased and prolonged blood levels of ampicillin and sulbactam. The concurrent administration of allopurinol and aampicillin increases substantially the incidence of rashes in patients receiving both drugs aas compared to patients receiving ampicillin alone. It is not known whether this potentiation oof ampicillin rashes is due to allopurinol or the hyperuricemia present in these ppatients. There are no data with Ampicillin and Sulbactam and allopurinol administered cconcurrently. Ampicillin and Sulbactam and aminoglycosides should not be reconstitute ttogether due to the in vitro inactivation of aminoglycosides by the ampicillin component of Ampicillin and Sulbactam for Injection.
Administration of Ampicillin and Sulbactam will result in high urine concentration of ampicillin. High urine concentrations of ampicillin may result in false positive reactions when testing for the presence of glucose in urine using Clinitest™, Benedict's Solution or Fehling's Solution. It is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix™) be used. Following administration of ampicillin to pregnant women, a transient decrease in plasma concentration of total conjugated eestriol, estriol-glucuronide, conjugated estrone and estradiol has been noted. This effect mmay also occur with Ampicillin and Sulbactam.
Long-term studies in animals have not been performed to evaluate carcinogenic or mutagenic potential.
Pregnancy Category B
Reproduction studies have been performed in mice, rats, and rabbits at doses up to ten (10) times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Ampicillin and Sulbactam for Injection. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. (See PRECAUTIONS, Drug/Laboratory Test Interactions section).
Studies in guinea pigs have shown that intravenous administration of ampicillin decreased the uterine tone, frequency of contractions, height of contractions, and duration of contractions. However, it is not known whether the use of Ampicillin and Sulbactam for Injection in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Low concentrations of ampicillin and sulbactam are excreted in the milk; therefore, caution should be exercised when Ampicillin and Sulbactam for Injection is administered to a nursing woman.
The safety and effectiveness of Ampicillin and Sulbactam for Injection have been established for pediatric patients one year of age and older for skin and skin structure infections as approved in adults. Use of Ampicillin and Sulbactam for Injection in pediatric ppatients is supported by evidence from adequate and well-controlled studies in adults wwith additional data from pediatric pharmacokinetic studies, a controlled clinical trial cconducted in pediatric patients and post-marketing adverse events surveillance (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, ADVERSE REACTIONS, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES sections). TThe safety and effectiveness of Ampicillin and Sulbactam for Injection have not been eestablished for pediatric patients for intra-abdominal infections.
ADVERSE REACTIONS
Ampicillin and Sulbactam for Injection is generally well tolerated. The following adverse reactions have been reported.
Pain at IM injection site - 16%
Pain at IV injection site - 3%
Thrombophlebitis - 3%
The most frequently reported adverse reactions were diarrhea in 3% of the patients and rash in less than 2% of the patients.
Additional systemic reactions reported in less than 1% of the patients were: itching, nausea, vomiting, candidiasis, fatigue, malaise, headache, chest pain, flatulence, abdominal distension, glossitis, urine retention, dysuria, edema, facial swelling, erythema, chills, tightness in throat, substernal pain, epistaxis and mucosal bleeding.
Available safety data for pediatric patients treated with Ampicillin and Sulbactam for Injection demonstrate a similar adverse events profile to those observed in adult patients. Additionally, atypical lymphocytosis has been observed in one pediatric patient receiving Ampicillin and Sulbactam for Injection.
Adverse Laboratory Changes
Adverse laboratory changes without regard to drug relationship that were reported during clinical trials were:
Hepatic: Increased AST (SGOT), ALT (SGPT), alkaline phosphatase, and LDH.
Hematologic: Decreased hemoglobin, hematocrit, RBC, WBC, neutrophils, lymphocytes, platelets and increased lymphocytes, monocytes, basophils, eosinophils, and platelets.
Blood Chemistry: Decreased serum albumin and total proteins.
Renal: Increased BUN and creatinine.
Urinalysis: Presence of RBCs and hyaline casts in urine.
The following adverse reactions have been reported with ampicillin-class antibiotics and can also occur with ampicillin and sulbactam for injection.
Gastrointestinal: Gastritis, stomatitis, black "hairy" tongue and enterocolitis. Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment. (See WARNINGS section).
Hypersensitivity Reactions: Urticaria, erythema multiforme, and an occasional case of exfoliative dermatitis have been reported. These reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, the drug should be discontinued, unless the opinion of the physician dictates otherwise. Serious and occasional fatal hypersensitivity (anaphylactic) reactions can occur with a penicillin. (See WARNINGS section).
Hematologic: In addition to the adverse laboratory changes listed above for ampicillin and sulbactam for injection, agranulocytosis has been reported during therapy with penicillins. All of these reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena. Some individuals have developed positive direct Coombs Tests during treatment with ampicillin and sulbactam for injection, as with other beta-lactam antibiotics.
To report SUSPECTED ADVERSE REACTIONS, contact WG Critical Care, LLC at 1-866-562-4708 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Neurological adverse reactions, including convulsions, may occur with the attainment of high CSF levels of beta-lactams. Ampicillin may be removed from circulation by hemodialysis. The molecular weight, degree of protein binding and pharmacokinetics profile of sulbactam suggest that this compound may also be removed by hemodialysis.
CLINICAL STUDIES
Skin and Skin Structure Infections in Pediatric Patients: Data from a controlled clinical trial conducted in pediatric patients provided evidence supporting the safety and efficacy of Ampicillin and Sulbactam for Injection for the treatment of skin and skin structure infections. Of 99 pediatric patients evaluable for clinical efficacy, 60 patients received a regimen containing intravenous ampicillin and sulbactam for injection, and 39 patients received a regimen containing intravenous cefuroxime. This trial demonstrated similar outcomes (assessed at an appropriate interval after discontinuation of all antimicrobial therapy) for Ampicillin and Sulbactam for Injection - and cefuroxime - treated patients:
| Therapeutic Regimen | Clinical Success | Clinical Failure |
| Ampicillin and Sulbactam | 51/60 (85%) | 9/60 (15%) |
| Cefuroxime | 34/39 (87%) | 5/39 (13%) |
Most patients received a course of oral antimicrobials following initial treatment with intravenous administration of parenteral antimicrobials. The study protocol required that the following three criteria be met prior to transition from intravenous to oral antimicrobial therapy: (1) receipt of a minimum of 72 hours of intravenous therapy; (2) no documented fever for prior 24 hours; and (3) improvement or resolution of the signs and symptoms of infection.
The choice of oral antimicrobial agent used in this trial was determined by susceptibility testing of the original pathogen, if isolated, to oral agents available. The course of oral antimicrobial therapy should not routinely exceed 14 days.
DOSAGE AND ADMINISTRATION
Ampicillin and Sulbactam for Injection may be administered by either the IV or the IM routes.
For IV administration, the dose can be given by slow intravenous injection over at least 10-15 minutes or can also be delivered in greater dilutions with 50-100 mL of a compatible diluent as an intravenous infusion over 15-30 minutes.
Ampicillin and Sulbactam for Injection may be administered by deep intramuscular injection (see DIRECTIONS FOR USE - Preparation for Intramuscular Injection section). The recommended adult dosage of Ampicillin and Sulbactam for Injection is 1.5 g (1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt) to 3 g (2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt) every six hours. This 1.5 to 3 g range represents the total of ampicillin content plus the sulbactam content of Ampicillin and Sulbactam for Injection, and corresponds to a range of 1 g ampicillin/0.5 g sulbactam to 2 g ampicillin/1 g sulbactam. The total dose of sulbactam should not exceed 4 grams per day.
Pediatric Patients 1 Year of Age or Older
The recommended daily dose of Ampicillin and Sulbactam for Injection in pediatric patients is 300 mg per kg of body weight administered via intravenous infusion in equally divided doses every 6 hours. This 300 mg/kg/day dosage represents the total ampicillin content plus the sulbactam content of Ampicillin and Sulbactam for Injection, and corresponds to 200 mg ampicillin/100 mg sulbactam per kg per day. The safety and efficacy of Ampicillin and Sulbactam for Injection administered via intramuscular injection in pediatric patients have not been established. Pediatric patients weighing 40 kg or more should be dosed according to adult recommendations, and the total dose of sulbactam should not exceed 4 grams per day. The course of intravenous therapy sshould not routinely exceed 14 days. In clinical trials, most children received a course of ooral antimicrobials following initial treatment with intravenous Ampicillin and Sulbactam ffor Injection (see CLINICAL STUDIES section).
Impaired Renal Function
In patients with impairment of renal function the elimination kinetics of ampicillin and sulbactam are similarly affected, hence the ratio of one to the other will remain constant whatever the renal function. The dose of Ampicillin and Sulbactam for Injection in such patients should be administered less frequently in accordance with the usual practice for ampicillin and according to the following recommendations:
| Creatinine Clearance (mL/min/1.73m 2 ) | Ampicillin/ Sulbactam Half-Life (Hours) |
Recommended
Ampicillin/Sulbactam Dosage |
| ≥ 30 | 1 | 1.5-3 g q 6 h-q 8h |
| 15-29 | 5 | 1.5-3 g q 12h |
| 5-14 | 9 | 1.5-3 g q 24h |
When only serum creatinine is available, the following formula (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.
| Males |
weight (kg) × (140 - age)
72 × serum creatinine |
| Females | 0.85 × above value |
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
COMPATIBILITY, RECONSTITUTION AND STABILITY
Ampicillin and Sulbactam for Injection sterile powder is to be stored at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature] prior to reconstitution.
When concomitant therapy with aminoglycosides is indicated, Ampicillin and Sulbactam for Injection and aminoglycosides should be reconstituted and administered separately, due to the in vitro inactivation of aminoglycosides by any of the aminopenicillins.
DIRECTIONS FOR USE
General Dissolution Procedures
Ampicillin and Sulbactam for Injection sterile powder for intravenous and intramuscular use may be reconstituted with any of the compatible diluents described in this insert. Solutions should be allowed to stand after dissolution to allow any foaming to dissipate in order to permit visual inspection for complete solubilization.
Preparation for Intravenous Use
1.5 g and 3 g Bottles: Ampicillin and Sulbactam for Injection sterile powder in piggyback units may be reconstituted directly to the desired concentrations using any of the following parenteral diluents. Reconstitution of Ampicillin and Sulbactam for Injection, at the sspecified concentrations, with these diluents provide stable solutions for the time periods iindicated in the following table: (After the indicated time periods, any unused portions of solutions should be discarded.)
| Diluent |
Maximum Concentration (mg/mL) Ampicillin And Sulbactam For Injection (Ampicillin/Sulbactam) |
Use Periods |
| Sterile Water for Injection | 45 (30/15) 45 (30/15) 30 (20/10) |
8 hrs at 25°C 48 hrs at 4°C 72 hrs at 4°C |
| 0.9% Sodium Chloride Injection | 45 (30/15) 45 (30/15) 30 (20/10) |
8 hrs at 25°C 48 hrs at 4°C 72 hrs at 4°C |
| 5% Dextrose Injection | 30 (20/10) 30 (20/10) 3 (2/1) |
2 hrs at 25°C 4 hrs at 4°C 4 hrs at 25°C |
| Lactated Ringer's Injection | 45 (30/15) 45 (30/15) |
8 hrs at 25°C 24 hrs at 4°C |
| M/6 Sodium Lactate Injection | 45 (30/15) 45 (30/15) |
8 hrs at 25°C 8 hrs at 4°C |
| 5% Dextrose in 0.45% Saline | 3 (2/1) 15 (10/5) |
4 hrs at 25°C 4 hrs at 4°C |
| 10% Invert Sugar | 3 (2/1) 30 (20/10) |
4 hrs at 25°C 3 hrs at 4°C |
If piggyback bottles are unavailable, standard vials of Ampicillin and Sulbactam sterile powder may be used. Initially, the vials may be reconstituted with Sterile Water for Injection to yield solutions containing 375 mg Ampicillin and Sulbactam for Injection per mL (250 mg ampicillin/125 mg sulbactam per mL). An appropriate volume should then be immediately diluted with a suitable parenteral diluent to yield solutions containing 3 to 45 mg Ampicillin and Sulbactam per mL (2 to 30 mg ampicillin/1 to 15 mg sulbactam per mL).
Preparation for Intramuscular Injection
1.5 g and 3 g Standard Vials: Vials for intramuscular use may be reconstituted with Sterile Water for Injection USP, 0.5% Lidocaine Hydrochloride Injection, USP or 2% Lidocaine Hydrochloride Injection, USP. Consult the following table for recommended volumes to be added to obtain solutions containing 375 mg Ampicillin and Sulbactam for injection per mL (250 mg ampicillin/125 mg sulbactam per mL). Note: Use only freshly prepared solutions and administer within one hour after preparation.
|
*There is sufficient excess present to allow withdrawal and administration of the stated volumes. |
||
| Ampicillin and Sulbactam for Injection Vial Size | Volume of Diluent to be Added |
Withdrawal Volume* |
| 1.5 g | 3.2 mL | 4 mL |
| 3 g | 6.4 mL | 8 mL |
Animal Pharmacology: While reversible glycogenosis was observed in laboratory animals, this phenomenon was dose- and time-dependent and is not expected to develop at the therapeutic doses and corresponding plasma levels attained during the relatively short periods of combined ampicillin/sulbactam therapy in man.
HOW SUPPLIED
Ampicillin and Sulbactam for Injection, USP is supplied as a sterile off-white dry powder in glass vials and piggyback bottles. The following packages are available:
NDC 44567-210-10 1.5 g vial – tray of 10 – equivalent to 1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt
NDC 44567-211-10 – 3 g vial – tray of 10 – equivalent to 2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt
Ampicillin and Sulbactam for Injection, USP is also supplied in a Pharmacy Bulk Package vial in the following package:
NDC 44567-212-01 – 15 g Pharmacy Bulk Package vial – equivalent to 10 g ampicillin as the sodium salt plus 5 g sulbactam as the sodium salt.
STORAGE: Before Reconstitution:
Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature].
REFERENCES
1. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard - 11th ed. CLSI document M02-A11, CLSI, 950 West Valley Road, Suite 2500, Wayne, PA 19087, 2012.
2. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. CLSI document M100-S22, 2012.
3. Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - 9th ed. CLSI document M07-A9, 2012.
4. Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard - 8th ed. CLSI document M11-A8, 2012.
Distributed by:
WG Critical Care, LLC
Paramus, NJ 07652
INS16014 01 02/2013
PRINCIPAL DISPLAY PANEL - 1.5 gram
NDC 44567-210-10
Ampicillin and Sulbactam for Injection, USP 1.5 gram/vial
For IV or IM Use
Rx only

PRINCIPAL DISPLAY PANEL - 3 gram
NDC 44567-211-10
Ampicillin and Sulbactam for Injection, USP 3 gram/vial
For IV or IM Use
Rx only

Ampicillin and SulbactamAmpicillin and Sulbactam INJECTION, POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ampicillin and SulbactamAmpicillin and Sulbactam INJECTION, POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||