AMOXICILLIN
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMOXICILLIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- Microbiology
- AMOXICILLIN INDICATIONS AND USAGE
- AMOXICILLIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMOXICILLIN ADVERSE REACTIONS
- OVERDOSAGE
- AMOXICILLIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
- PACKAGE LABEL - AMOXICILLIN 875 MG TABLET
FULL PRESCRIBING INFORMATION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of amoxicillin and other antibacterial drugs, amoxicillin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
AMOXICILLIN DESCRIPTION
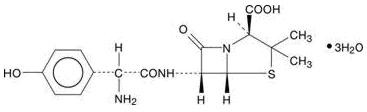
Formulations of amoxicillin tablets, USP contain amoxicillin, a semisynthetic antibiotic, an analog of ampicillin, with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms. Chemically, it is (2S,5R,6R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate. It may be represented structural formula as:
 1619352
1619352CLINICAL PHARMACOLOGY
Amoxicillin is stable in the presence of gastric acid and is rapidly absorbed
after oral administration. The effect of food on the absorption of amoxicillin
from the tablets and suspension of amoxicillin has been partially investigated.
The 400 mg and 875 mg formulations have been studied only when administered at
the start of a light meal. However, food effect studies have not been performed
with the 200 mg and 500 mg formulations. Amoxicillin diffuses readily into most
body tissues and fluids, with the exception of brain and spinal fluid, except
when meninges are inflamed. The half-life of amoxicillin is 61.3 minutes. Most
of the amoxicillin is excreted unchanged in the urine; its excretion can be
delayed by concurrent administration of probenecid. In blood serum, amoxicillin
is approximately 20% protein-bound.
Orally administered doses of 250 mg
and 500 mg amoxicillin capsules result in average peak blood levels 1 to 2 hours
after administration in the range of 3.5 mcg/mL to 5 mcg/mL and 5.5 mcg/mL to
7.5 mcg/mL, respectively.
Mean amoxicillin pharmacokinetic parameters
from an open, two-part, single-dose crossover bioequivalence study in 27 adults
comparing 875 mg of amoxicillin with 875 mg of amoxicillin/clavulanate potassium
showed that the 875 mg tablet of amoxicillin produces an AUC0-∞ of 35.4 ± 8.1 mcg•hr/mL and a Cmax
of 13.8 ± 4.1 mcg/mL. Dosing was at the start of a light meal following an
overnight fast.
Orally administered doses of amoxicillin suspension, 125
mg/5 mL and 250 mg/5 mL, result in average peak blood levels 1 to 2 hours after
administration in the range of 1.5 mcg/mL to 3 mcg/mL and 3.5 mcg/mL to 5
mcg/mL, respectively.
Oral administration of single doses of 400 mg
chewable tablets and 400 mg/5 mL suspension of amoxicillin to 24 adult
volunteers yielded comparable pharmacokinetic data:
| Dose * |
|
AUC 0-∞ (mcg•hr/mL) |
|
Cmax (mcg/mL)† |
| Amoxicillin |
|
Amoxicillin |
|
Amoxicillin |
|
|
|
(±S.D.) |
|
(±S.D.) |
|
|
|
|
|
|
| 400 mg (5 mL of suspension) |
|
17.1 (3.1) |
|
5.92 (1.62) |
| 400 mg (1 chewable tablet) |
|
17.9 (2.4) |
|
5.18 (1.64) |
Microbiology
Amoxicillin is similar to ampicillin in its bactericidal action against
susceptible organisms during the stage of active multiplication. It acts through
the inhibition of biosynthesis of cell wall mucopeptide. Amoxicillin has been
shown to be active against most strains of the following microorganisms, both
in vitro and in clinical infections as described in
the
INDICATIONS AND USAGE
section.
Aerobic Gram-Positive Microorganisms
Enterococcus faecalis
Staphylococcus spp.* (β-lactamase–negative strains
only)
Streptococcus pneumoniae
Streptococcus spp. (α- and β-hemolytic strains
only)
*Staphylococci which are susceptible to amoxicillin but resistant
to methicillin/oxacillin should be considered as resistant to
amoxicillin.
Aerobic Gram-Negative Microorganisms
Escherichia coli (β-lactamase–negative strains
only)
Haemophilus influenzae (β-lactamase–negative
strains only)
Neisseria gonorrhoeae
(β-lactamase–negative strains only)
Proteus
mirabilis (β-lactamase–negative strains only)
Helicobacter
Helicobacter pylori
Susceptibility
Tests
Dilution Techniques
Quantitative
methods are used to determine antimicrobial minimum inhibitory concentrations
(MICs). These MICs provide estimates of the susceptibility of bacteria to
antimicrobial compounds. The MICs should be determined using a standardized
procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum
concentrations and standardized concentrations of ampicillin powder. Ampicillin is sometimes used to predict
susceptibility of S. pneumoniae to amoxicillin;
however, some intermediate strains have been shown to be susceptible to
amoxicillin. Therefore, S. pneumoniae susceptibility
should be tested using amoxicillin powder. The MIC values should be interpreted
according to the following criteria:
For Gram-Positive Aerobes
Enterococcus
| MIC
(mcg/mL) |
Interpretation
|
| ≤8 |
Susceptible (S) |
| ≥16 |
Resistant (R) |
Staphylococcusa
| MIC
(mcg/mL) |
Interpretation |
| ≤0.25 |
Susceptible (S) |
| ≥0.5 |
Resistant (R) |
Streptococcus S. pneumoniae
| MIC
(mcg/mL) |
Interpretation |
| ≤0.25 |
Susceptible (S) |
| 0.5 to 4 |
Intermediate (I) |
| ≥8 |
Resistant (R) |
S. pneumoniaeb
Amoxicillin
| MIC
(mcg/mL) |
Interpretation |
| ≤2 |
Susceptible (S) |
| 4 |
Intermediate (I) |
| ≥8 |
Resistant (R) |
NOTE:
Enterobacteriaceae
| MIC
(mcg/mL) |
Interpretation |
| ≤8 |
Susceptible (S) |
| 16 |
Intermediate (I) |
| ≥32 |
Resistant (R) |
H. influenzaec
| MIC
(mcg/mL) |
Interpretation |
| ≤1 |
Susceptible (S) |
| 2 |
Intermediate (I) |
| ≥4 |
Resistant (R) |
H. influenzae Haemophilus1
ampicillin
|
Microorganism |
MIC Range
(mcg/mL) |
|
E.
coli ATCC 25922 |
2 to 8 |
|
E. faecalis
ATCC 29212 |
0.5 to 2 |
|
H. influenzae
ATCC 49247d
|
2 to 8 |
|
S.
aureus ATCC 29213 |
0.25 to
1 |
amoxicillin
|
Microorganism
|
MIC Range
(mcg/mL)
|
|
S. pneumoniae
ATCC 49619e
|
0.03 to
0.12 |
H. influenzae1
S. pneumoniae
Diffusion Techniques
2S. pneumoniaeampicillin
Enterococcus
| Zone
Diameter (mm)
|
Interpretation
|
| ≥17 |
Susceptible (S) |
| ≤16 |
Resistant (R) |
Staphylococcusf
| Zone
Diameter (mm)
|
Interpretation
|
| ≥29 |
Susceptible (S) |
| ≤28 |
Resistant (R) |
-
| Zone
Diameter (mm) |
Interpretation |
| ≥26 |
Susceptible (S) |
| 19 to 25 |
Intermediate (I) |
| ≤18 |
Resistant (R) |
NOTE: Spneumoniae
S. pneumoniae
S. pneumoniae S. pneumoniae
| Zone
Diameter (mm) |
Interpretation |
| ≥17 |
Susceptible (S) |
| 14 to 16 |
Intermediate (I) |
| ≤13 |
Resistant (R) |
H. influenzaeg
| Zone
Diameter (mm) |
Interpretation |
| ≥22 |
Susceptible (S) |
| 19 to 21 |
Intermediate (I) |
| ≤18 |
Resistant (R) |
H. influenzaeHaemophilus2
ampicillin
|
Microorganism
|
Zone Diameter
(mm)
|
|
E.
coli ATCC 25922 |
16 to 22 |
|
H.
influenzae ATCC 49247h
|
13 to 21 |
|
S.
aureus ATCC 25923 |
27 to
35 |
oxacillin
|
Microorganism
|
Zone Diameter
(mm) |
|
S.
pneumoniae ATCC 49619i
|
8 to
12 |
H. influenzae2
S. pneumoniae2
Helicobacter pylori
In vitroH. pylori
AMOXICILLIN INDICATIONS AND USAGE
Amoxicillin is indicated in the treatment of infections due to susceptible (ONLY
β-lactamase–negative) strains of the designated microorganisms in the conditions
listed below:
Infections of the ear, nose, and throat
– due to Streptococcus spp. (α- and
β-hemolytic strains only), S. pneumoniae, Staphylococcus spp., or H.
influenzae.
Infections of the genitourinary tract
– due to E. coli, P. mirabilis, or E. faecalis.
Infections of the skin
and skin structure – due to Streptococcus spp.
(α- and β-hemolytic strains only), Staphylococcus
spp., or E. coli.
Infections
of the lower respiratory tract – due to Streptococcus spp. (α- and β-hemolytic strains only), S. pneumoniae, Staphylococcus spp., or H. influenzae.
Gonorrhea, acute
uncomplicated (ano-genital and urethral infections) – due to N. gonorrhoeae (males and females).
H. pylori
eradication to reduce the risk of duodenal ulcer
recurrence
Triple Therapy
Amoxicillin/clarithromycin/lansoprazole
Amoxicillin,
in combination with clarithromycin plus lansoprazole as triple therapy, is
indicated for the treatment of patients with H.
pylori infection and duodenal ulcer disease (active or 1-year history of
a duodenal ulcer) to eradicate H. pylori. Eradication
of H. pylori has been shown to reduce the risk of
duodenal ulcer recurrence. (See
CLINICAL
STUDIES
and
DOSAGE AND
ADMINISTRATION
.)
Dual Therapy
Amoxicillin/lansoprazole
Amoxicillin, in
combination with lansoprazole delayed-release capsules as dual therapy, is
indicated for the treatment of patients with H.
pylori infection and duodenal ulcer disease (active or 1-year history of
a duodenal ulcer) who are either allergic or intolerant to
clarithromycin or in whom resistance to clarithromycin is known or
suspected. (See the clarithromycin package insert, MICROBIOLOGY.)
Eradication of H. pylori has been shown to reduce the
risk of duodenal ulcer recurrence. (See
CLINICAL STUDIES
and
DOSAGE AND ADMINISTRATION
.)
To reduce the
development of drug-resistant bacteria and maintain the effectiveness of
amoxicillin and other antibacterial drugs, amoxicillin should be used only to
treat or prevent infections that are proven or strongly suspected to be caused
by susceptible bacteria. When culture and susceptibility information are
available, they should be considered in selecting or modifying antibacterial
therapy. In the absence of such data, local epidemiology and susceptibility
patterns may contribute to the empiric selection of therapy.
Indicated
surgical procedures should be performed.
AMOXICILLIN CONTRAINDICATIONS
A history of allergic reaction to any of the penicillins is a contraindication.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE
BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE
FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL
PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A
HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO
MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF
PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED
WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH AMOXICILLIN, CAREFUL INQUIRY
SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS,
CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, AMOXICILLIN
SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT
WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING
INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile associated diarrhea (CDAD)
has been reported with use of nearly all antibacterial agents, including
amoxicillin, and may range in severity from mild diarrhea to fatal colitis.
Treatment with antibacterial agents alters the normal flora of the colon leading
to overgrowth of C. difficile.
C. difficile produces toxins A and B which
contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as
these infections can be refractory to antimicrobial therapy and may require
colectomy. CDAD must be considered in all patients who present with diarrhea
following antibiotic use. Careful medical history is necessary since CDAD has
been reported to occur over two months after the administration of antibacterial
agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not
directed against C. difficile may need to be
discontinued. Appropriate fluid and electrolyte management, protein
supplementation, antibiotic treatment of C.
difficile, and surgical evaluation should be instituted as clinically
indicated.
PRECAUTIONS
General
Laboratory Tests
Drug Interactions
in vitro
Drug/Laboratory Test Interactions
®®
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of amoxicillin alone have not been conducted; however, the following information is available from tests on a 4:1 mixture of amoxicillin and potassium clavulanate. Amoxicillin and potassium clavulanate was non-mutagenic in the Ames bacterial mutation assay, and the yeast gene conversion assay. Amoxicillin and potassium clavulanate was weakly positive in the mouse lymphoma assay, but the trend toward increased mutation frequencies in this assay occurred at doses that were also associated with decreased cell survival. Amoxicillin and potassium clavulanate was negative in the mouse micronucleus test, and in the dominant lethal assay in mice. Potassium clavulanate alone was tested in the Ames bacterial mutation assay and in the mouse micronucleus test, and was negative in each of these assays. In a multi-generation reproduction study in rats, no impairment of fertility or other adverse reproductive effects were seen at doses up to 500 mg/kg (approximately 3 times the human dose in mg/m2).
Pregnancy
Labor and Delivery
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION: Neonates and Infants
Geriatric Use
Information for Patients
AMOXICILLIN ADVERSE REACTIONS
As with other penicillins, it may be expected that untoward reactions will be
essentially limited to sensitivity phenomena. They are more likely to occur in
individuals who have previously demonstrated hypersensitivity to penicillins and
in those with a history of allergy, asthma, hay fever, or urticaria. The
following adverse reactions have been reported as associated with the use of
penicillins:
Infections and
Infestations
Mucocutaneous candidiasis.
Gastrointestinal
Nausea, vomiting, diarrhea,
black hairy tongue, and hemorrhagic/pseudomembranous colitis.
Onset of
pseudomembranous colitis symptoms may occur during or after antibiotic
treatment. (See
WARNINGS
.)
Hypersensitivity Reactions
Anaphylaxis (See
WARNINGS
.)
Serum
sickness–like reactions, erythematous maculopapular rashes, erythema multiforme,
Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis,
acute generalized exanthematous pustulosis, hypersensitivity vasculitis and
urticaria have been reported.
NOTE: These
hypersensitivity reactions may be controlled with antihistamines and, if
necessary, systemic corticosteroids. Whenever such reactions occur, amoxicillin
should be discontinued unless, in the opinion of the physician, the condition
being treated is life-threatening and amenable only to amoxicillin therapy.
Liver
A moderate rise in AST (SGOT) and/or ALT
(SGPT) has been noted, but the significance of this finding is unknown. Hepatic
dysfunction including cholestatic jaundice, hepatic cholestasis and acute
cytolytic hepatitis have been reported.
Renal
Crystalluria has also been reported (see
OVERDOSAGE
).
Hemic and Lymphatic Systems
Anemia, including
hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia,
leukopenia, and agranulocytosis have been reported during therapy with
penicillins. These reactions are usually reversible on discontinuation of
therapy and are believed to be hypersensitivity phenomena.
Central Nervous System
Reversible
hyperactivity, agitation, anxiety, insomnia, confusion, convulsions, behavioral
changes, and/or dizziness have been reported rarely.
Miscellaneous
Tooth discoloration (brown,
yellow, or gray staining) has been rarely reported. Most reports occurred in
pediatric patients. Discoloration was reduced or eliminated with brushing or
dental cleaning in most cases.
Combination Therapy with
Clarithromycin and Lansoprazole
In clinical trials using
combination therapy with amoxicillin plus clarithromycin and lansoprazole, and
amoxicillin plus lansoprazole, no adverse reactions peculiar to these drug
combinations were observed. Adverse reactions that have occurred have been
limited to those that had been previously reported with amoxicillin,
clarithromycin, or lansoprazole.
Triple Therapy
Amoxicillin/Clarithromycin/Lansoprazole
The most
frequently reported adverse events for patients who received triple therapy were
diarrhea (7%), headache (6%), and taste perversion (5%). No treatment-emergent
adverse events were observed at significantly higher rates with triple therapy
than with any dual therapy regimen.
Dual Therapy
Amoxicillin/Lansoprazole
The most frequently
reported adverse events for patients who received amoxicillin three times daily
plus lansoprazole three times daily dual therapy were diarrhea (8%) and headache
(7%). No treatment-emergent adverse events were observed at significantly higher
rates with amoxicillin three times daily plus lansoprazole three times daily
dual therapy than with lansoprazole alone.
For more information on
adverse reactions with clarithromycin or lansoprazole, refer to their package
inserts, ADVERSE REACTIONS.
OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically, and
institute supportive measures as required. If the overdosage is very recent and
there is no contraindication, an attempt at emesis or other means of removal of
drug from the stomach may be performed. A prospective study of 51 pediatric
patients at a poison-control center suggested that overdosages of less than 250
mg/kg of amoxicillin are not associated with significant clinical symptoms and
do not require gastric emptying.3
Interstitial
nephritis resulting in oliguric renal failure has been reported in a small
number of patients after overdosage with amoxicillin.
Crystalluria, in
some cases leading to renal failure, has also been reported after amoxicillin
overdosage in adult and pediatric patients. In case of overdosage, adequate
fluid intake and diuresis should be maintained to reduce the risk of amoxicillin
crystalluria.
Renal impairment appears to be reversible with cessation
of drug administration. High blood levels may occur more readily in patients
with impaired renal function because of decreased renal clearance of
amoxicillin. Amoxicillin may be removed from circulation by hemodialysis.
AMOXICILLIN DOSAGE AND ADMINISTRATION
Neonates and Infants Aged ≤12 Weeks (≤3 Months)
Adults and Pediatric Patients >3 Months
| Infection |
|
Severity * |
|
Usual Adult Dose | Usual Dose for Children |
|
|
|
|
|
|
>3 Months†‡ |
|
|
|
|
|
|
|
| Ear/Nose/Throat |
|
Mild/Moderate |
|
500 mg every 12 hours | 25 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
250 mg every 8 hours | or |
|
|
|
|
|
|
20 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
|
|
|
Severe |
|
875 mg every 12 hours | 45 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
500 mg every 8 hours | or |
|
|
|
|
|
|
40 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
| Lower Rispiratory Tract |
|
Mild/Moderate or Severe |
|
875 mg every 12 hours | 45 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
500 mg every 8 hours | or |
|
|
|
|
|
|
40 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
| Skin/Skin Structure |
|
Mild/Moderate |
|
500 mg every 12 hours | 25 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
250 mg every 8 hours | or |
|
|
|
|
|
|
20 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
|
|
|
Severe |
|
875 mg every 12 hours | 45 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
500 mg every 8 hours | or |
|
|
|
|
|
|
40 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
| Genitourinary Tract |
|
Mild/Moderate |
|
500 mg every 12 hours | 25 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
250 mg every 8 hours | or |
|
|
|
|
|
|
20 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
|
|
|
Severe |
|
875 mg every 12 hours | 45 mg/kg/day in divided |
|
|
|
|
|
or | doses every 12 hours |
|
|
|
|
|
500 mg every 8 hours | or |
|
|
|
|
|
|
40 mg/kg/day in divided |
|
|
|
|
|
|
doses every 8 hours |
|
|
|
|
|
|
|
| Gonorrhea Acute, |
|
|
|
3 grams as single oral | Prepubertal children: |
| uncomplicated |
|
|
|
dose | 50 mg/kg amoxicillin, |
| ano-genital and |
|
|
|
|
combined with 25 mg/kg |
| urethral infections |
|
|
|
|
probenecid as a single |
| in males and females |
|
|
|
|
dose. |
|
|
|
|
|
|
NOTE: SINCE |
|
|
|
|
|
|
PROBENECID IS |
|
|
|
|
|
|
CONTRAINDICATED |
|
|
|
|
|
|
IN CHILDREN UNDER |
|
|
|
|
|
|
2 YEARS, DO NOT USE |
|
|
|
|
|
|
THIS REGIMEN IN |
|
|
|
|
|
|
THESE CASES. |
†
‡
PRECAUTIONS: Laboratory Tests
General
Streptococcus pyogenes
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Amoxicillin/clarithromycin/lansoprazole
INDICATIONS AND USAGE
Amoxicillin/lansoprazole
INDICATIONS AND USAGE
Dosing Recommendations for Adults with Impaired Renal Function
There are currently no dosing recommendations for pediatric patients with impaired renal function.
HOW SUPPLIED
Amoxicillin Tablets, USP contains 875 mg amoxicillin as
the trihydrate.
875 mg Tablet
Pink
colored, capsule shaped, film coated tablets debossed with “A” on one side and
with a score line in between “6” and “7” on the other
side.
Bottles of 20 NDC
59762-1050-2
Bottles of 100 NDC
59762-1050-5
Store at 20° to 25°C (68° to 77°F);
excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room
Temperature].
Dispense in a tight container.
CLINICAL STUDIES
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
H. pyloriH. pylori
H. pylori
H. pylori
H. pylori Eradication Rates – Triple Therapy (amoxicillin/clarithromycin/lansoprazole) Percent of Patients Cured [95% Confidence Interval] (Number of Patients)
| Study |
|
Triple Therapy | Triple Therapy |
|
|
|
Evaluable Analysis * | Intent-to-Treat Analysis † |
|
|
|
|
|
| Study 1 |
|
92‡ | 86‡ |
|
|
|
[80 - 97.7] | [73.3 - 93.5] |
|
|
|
(n = 48) | (n = 55) |
|
|
|
|
|
| Study 2 |
|
86§ | 83§ |
|
|
|
[75.7 - 93.6] | [72 - 90.8] |
|
|
|
(n = 66) | (n = 70) |
H. pylori®
† H. pylori
‡
§
H. pylori Eradication Rates – Dual Therapy (amoxicillin/lansoprazole) Percent of Patients Cured [95% Confidence Interval] (Number of Patients)
| Study |
|
Dual Therapy | Dual Therapy |
|
|
|
Evaluable Analysis * | Intent-to-Treat Analysis † |
|
|
|
|
|
| Study 1 |
|
77‡ | 70‡ |
|
|
|
[62.5 - 87.2] | [56.8 - 81.2] |
|
|
|
(n = 51) | (n = 60) |
|
|
|
|
|
| Study 2 |
|
66§ | 61§ |
|
|
|
[51.9 - 77.5] | [48.5 - 72.9] |
|
|
|
(n = 58) | (n = 67) |
† H. pylori
‡
§
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Fourth Edition; Approved Standard NCCLS Document M7-A4, Vol. 17, No. 2. NCCLS, Wayne, PA, January 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests – Sixth Edition; Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1. NCCLS, Wayne, PA, January 1997.
- Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988;30:66-67.
GREENSTONE® BRAND
Distributed by:
Greenstone LLC
PACKAGE LABEL - AMOXICILLIN 875 MG TABLET

AMOXICILLINAMOXICILLIN TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||