Alcortin A
Primus Pharmaceuticals
Sonar Products, Inc
ALCORTIN® A gel
FULL PRESCRIBING INFORMATION: CONTENTS*
- ALCORTIN A DESCRIPTION
- CLINICAL PHARMACOLOGY
- ALCORTIN A INDICATIONS AND USAGE
- ALCORTIN A CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- ALCORTIN A ADVERSE REACTIONS
- ALCORTIN A DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 24 Pack Carton
FULL PRESCRIBING INFORMATION
Prescribing Information
ALCORTIN A DESCRIPTION
Each gram of Alcortin® A contains 2.0% (20 mg) Hydrocortisone Acetate and 1.0% (10 mg) Iodoquinol. Also contains 1.0% (10 mg) Aloe polysaccharide
#6,436,679; #6,271,214; #6,133,440; #5,708,038; patent pending
Iodoquinol
Iodoquinol is an antifungal and antibacterial agent. Chemically, Iodoquinol is [5,7-diiodo-8-quinolinol] with the molecular formula (C9H5I2NO) and is represented by the following structural formula:
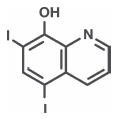
Hydrocortisone Acetate
Hydrocortisone acetate is an anti-inflammatory and antipruritic agent. Chemically, hydrocortisone acetate is [Pregn-4-ene-3, 20-dione, 11, 17, 21- trihydroxy-, (11β)-] with the molecular formula (C21H30O5) and is represented by the following structural formula:
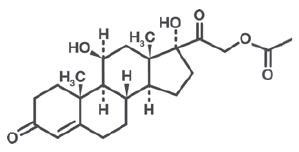
CLINICAL PHARMACOLOGY
Hydrocortisone Acetate has anti-inflammatory, antipruritic and vasoconstrictive properties. While the mechanism of anti-inflammatory activity is unclear, there is evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in humans. Iodoquinol has both antifungal and antibacterial properties.
Pharmacokinetics
The extent of percutaneous absorption of topical steroids is determined by many factors including the vehicle, the integrity of the epidermal barrier and the use of occlusive dressings. Hydrocortisone acetate can be absorbed from normal intact skin. Inflammation and/or other inflammatory disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Once absorbed through the skin, hydrocortisone acetate is metabolized in the liver and most body tissue to hydrogenated and degraded forms such as tetrahydrocortisone and tetrahydrocortisol. These are excreted in the urine, mainly conjugated as glucuronides, together with a very small proportion of unchanged hydrocortisone acetate. There are no data available regarding the percutaneous absorption of iodoquinol; however, following oral administration, 3-5% of the dose was recovered in the urine as a glucuronide.
ALCORTIN A INDICATIONS AND USAGE
Based on a review of a related drug by the National Research Council and subsequent FDA classification for that drug, the indications are as follows: "Possibly" Effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); monliasis; intertrigo. Final classification of the less-than-effective indications requires further investigation.
ALCORTIN A CONTRAINDICATIONS
Alcortin A is contraindicated in those patients with a history of hypersensitivity to hydrocortisone acetate, iodoquinol, aloe vera, glycine, histidine, lysine, palmitic acid or any other components of the preparation.
WARNINGS AND PRECAUTIONS
For external use only. Keep away from eyes. If irritation develops, the use of Alcortin A should be discontinued and appropriate therapy instituted. Staining of the skin, hair and fabrics may occur. Not intended for use on infants or under diapers or occlusive dressings. If extensive areas are treated or if the occlusive dressing technique is used, the possibility exists of increased systemic absorption of the corticosteroid, and suitable precautions should be taken. Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings. Iodoquinol may be absorbed through the skin and interfere with thyroid function tests. If such tests are contemplated, wait at least one month after discontinuance of therapy to perform these tests. The ferric chloride test for phenylketonuria (PKU) can yield a false positive result if iodoquinol is present in the diaper or urine. Prolonged use may result in overgrowth of non-susceptible organisms requiring appropriate therapy. Keep out of reach of children. Burning, itching, irritation and dryness have been reported infrequently following the use of topical corticosteroids.
Carcinogenesis, Mutagenisis and Impairment of Fertility
Long term animal studies have not been performed to evaluate the carcinogenic potential of the effect on fertility of hydrocortisone or iodoquinol. In vitro studies to determine mutagenicity with hydrocortisone have revealed negative results. Mutagenicity studies have not been performed with iodoquinol.
Pregnancy Category C
Animal reproductive studies have not been conducted with Alcortin A. It is not known whether Alcortin A can cause fetal harm when administered to pregnant women or can affect reproductive capacity. Alcortin A should be given to pregnant women only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Alcortin A is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients under the age of 12 have not been established.
ALCORTIN A ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids. These reactions are listed in an approximate decreasing order of occurrence. Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infections, skin atrophy, striae and miliaria.
ALCORTIN A DOSAGE AND ADMINISTRATION
Apply to affected area 3-4 times daily in accordance with physician's directions or as directed otherwise by a physician.
HOW SUPPLIED
FORM
48.0 gram carton of 24-count of 2.0 gram gel individual packs
NDC #68040-705-13
2.0 gram gel individual pack
NDC # 68040-705-02
10 count carton of 2.0 gram gel sample packs - not for resale
NDC # 68040-705-08
Each 2.0 gram gel pack contains multiple doses depending on the surface area treated.
STORAGE
Store at room temperature 15º-30ºC (59º-86ºF).
Keep tightly closed.
Rx only
www.alcortin.com
Distributed by:
Primus Pharmaceuticals, Inc.
Scottsdale, AZ 85251
www.primusrx.com
Manufactured by:
Sonar Products, Inc.
Carlstadt, NJ 07072
© 2007 Primus Pharmaceuticals, Inc. All rights reserved.
ISS. 1207 #00499
PRINCIPAL DISPLAY PANEL - 24 Pack Carton
NDC 68040-705-13
Rx only
Alcortin® A Gel
1% iodoquinol•2% hydrocortisone acetate
1% aloe polysaccharides
Anti-fungal • Anti-bacterial • Anti-inflammatory
24
PACK
Hygienic • Convenient
& Portable
Contains moisturizers • For dermatological use only
Biopeptide Aloe Complex™
Deeper Penetration
Patented Formula
Net Wt. 48.0 g (1.69 oz.) • 24 packs of 2.0g (0.07 oz.) each
Each pack contains multiple doses
(depending on the surface area treated)

Alcortin AHYDROCORTISONE ACETATE, ALOE VERA LEAF and Iodoquinol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||