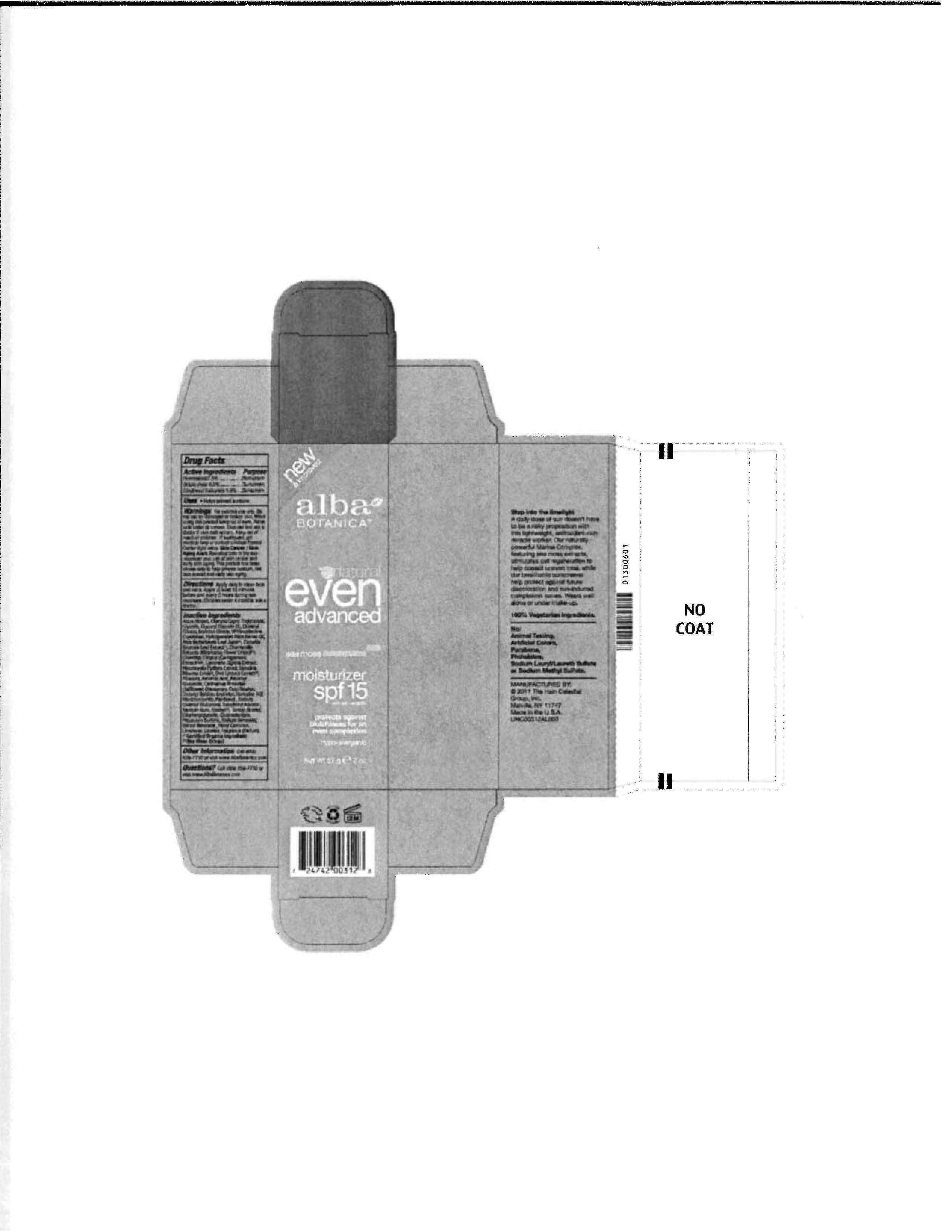
Alba Botanica Natural Even Advanced Sea Moss Moisturizer SPF15
The Hain Celestial Group, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Homosalate 7.5%
Octocrylene 5.0%
Ethylhexyl Salicylate 5.0%
Purpose
Sunscreen
Uses
- Helps prevent sunburn
For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if skin rash occurs.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Skin protection measures:
Aqua (Water), Caprylic/Capric Triglyceride, Glycerin, Glyceryl Stearate SE, Cetearyl Olivate, Sorbitan Olivate, VP/Hexadecene Copolymer, Hydrogenated Palm Kernel Oil, Aloe Barbadensis Leaf Juice (1), Chondrus Crispus (Sarrageenan) Extract (1) (2), Camellia Sinensis Leaf Extract (1), Chamomilla Recuitita (Matricaria) Flower Extract (1), Laminaria Digitata Extract, Macrocystis Purifera Extract, Spirulina Maxima Extract, Ulva Lactuca Extract (1), Cetyl Alcohol, Allantoin Ascorbic Acid, Ascorbyl Glucoside, Carthamus Tinctorius (Safflower) Oleosomes, Diacetyl Boldine, Erythritol, Homarine HCl, Montmorillonite, Panthenol ,Sodium Stearoyl Glutamate, Ethylhexylglycerin, Gluconolactone, Tocopheryl Acetate, Xanthan Gum, Alcohol (1), Benzyl Alcohol, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Benzyl Benzoate, Hexyl Cinnamal, Limonene, Linalool, Fragrance (Parfum).
(1) Certified Organic Ingredient
(2) Sea Moss Extract
888-659-7730, www.AlbaBotanica.com

Alba Botanica Natural Even Advanced Sea Moss Moisturizer SPF15Homosalate, Octocrylene, Ethylhexyl Salicylate LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||