Zostrix Joint and Arthritis Pain Relief
Health Care Products
Hi-Tech Pharmacal Co., Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Zostrix Joint and Arthritis Pain Relief Uses
- Warnings
- Directions
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Capsaicin 0.025%
Menthol 2.0%
Purpose
Topical Analgesic
Topical Analgesic
Keep Out of Reach of Children
Zostrix Joint and Arthritis Pain Relief Uses
For the temporary relief of minor aches and pains of the muscle joints associated with
- strains
- sprains
- bruises
- arthritis
Warnings
For external use only.
Do not apply to wounds or to damaged or irritated skin.
When using this product
- you may experience a burning sensation which is normal and related to the way the product works. With regular use, this sensation generally diminishes.
- avoid contact with eyes. Do not get it on mucous membranes, into eyes, or on contact lenses. If this occurs, rinse the affected area thoroughly with water.
- do not apply immediately before or after activities such as bathing, swimming, sun bathing, or strenuous exercise
- do not apply heat to the treated areas immediately before or after use
- do not tightly wrap or bandage the treated area
- avoid inhaling airborne material from dried residue. This can result in coughing, sneezing, tearing, throat or respiratory irritation.
Stop use and ask a doctor if
- condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
- blistering occurs
- difficulty breathing or swallowing occurs
- severe burning persists
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- for persons under 18 years of age, ask a doctor before using
- apply a thin film of cream to the affected area and gently rub in until fully absorbed
- for optimum relief, apply 3 to 4 times daily
- best results typically occur after 2 to 4 weeks of continuous use
- unless treating hands, wash hands thoroughly with soap and water immediately after use
- see package insert for more information
Store at 15°-30°C (59°-86°F).
Inactive ingredients
benzyl alcohol, cetearyl alcohol, ceteareth-20, cetyl alcohol, glyceryl stearate, isopropyl myristate, PEG-100 stearate, petrolatum, sorbitol & water.
Call 1-866-263-9003, Mon. - Thurs. 9:00 am - 5:00 pm EST, Fri. 9:00 am - 2:30 pm EST. Serious side effects associated with the use of this product may be reported to this number.
Package/Label Principal Display Panel
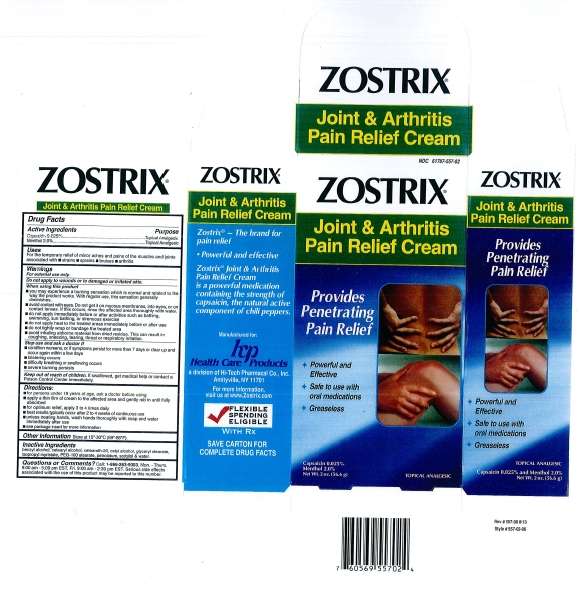
NDC 61787-557-02
Zostrix Joint & Arthritis Pain Relief Cream
Provides Pen etrating Pain Relief
- Powerful and Effective
- Safe to use with oral medications
- Greaseless
Capsaicin 0.025%
Menthol 2.0%
Topical Analgesic
Net Wt. 2 oz. (56.6 g)
Zostrix Joint and Arthritis Pain ReliefCapsaicin and Menthol CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||