Zorbtive
EMD Serono, Inc.
EMD Serono, Inc.
Zorbtive® [somatropin (rDNA origin) for injection]
FULL PRESCRIBING INFORMATION: CONTENTS*
- ZORBTIVE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ZORBTIVE INDICATIONS AND USAGE
- ZORBTIVE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ZORBTIVE ADVERSE REACTIONS
- OVERDOSAGE
- ZORBTIVE DOSAGE AND ADMINISTRATION
- Patient Package Insert
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
ZORBTIVE DESCRIPTION
Zorbtive® [somatropin (rDNA origin) for injection] is a human growth hormone (hGH) produced by recombinant DNA technology. Zorbtive® has 191 amino acid residues and a molecular weight of 22,125 daltons. Its amino acid sequence and structure are identical to the dominant form of human pituitary GH. Zorbtive® is produced by a mammalian cell line (mouse C127) that has been modified by the addition of the hGH gene. Zorbtive® is secreted directly through the cell membrane into the cell-culture medium for collection and purification.
Zorbtive® is a highly purified preparation. Biological potency is determined by measuring the increase in the body weight induced in hypophysectomized rats.
Zorbtive® is available in 8.8 mg vials for multi-dose administration. Each 8.8 mg vial contains 8.8 mg somatropin, 60.19 mg sucrose and 2.05 mg phosphoric acid. The pH is adjusted with sodium hydroxide or phosphoric acid to give a pH of 7.4 to 8.5 after reconstitution.
CLINICAL PHARMACOLOGY
Zorbtive® [somatropin (rDNA origin) for injection] is an anabolic and anticatabolic agent which exerts its influence by interacting with specific receptors on a variety of cell types including myocytes, hepatocytes, adipocytes, lymphocytes, and hematopoietic cells. Some, but not all of its effects, are mediated by insulin-like growth factor-1 (IGF-1).
MECHANISM OF ACTION – IN SHORT BOWEL SYNDROME (SBS) PATIENTS
Intestinal mucosa contains receptors for growth hormone and for insulin-like growth factor-1 (IGF-1), which is known to mediate many of the cellular actions of growth hormone. Thus, the actions of growth hormone on the gut may be direct or mediated via the local or systemic production of IGF-1.
In human clinical studies the administration of growth hormone has been shown to enhance the transmucosal transport of water, electrolytes, and nutrients.
PHARMACOKINETICS
Subcutaneous Absorption: The absolute bioavailability of Zorbtive® [somatropin (rDNA origin) for injection] after subcutaneous administration of a formulation not equivalent to the marketed formulation was determined to be 70-90%. The t½ (Mean ± SD) after subcutaneous administration is significantly longer than that seen after intravenous administration in normal male volunteers down-regulated with somatostatin (3.94 ± 3.44 hrs. vs. 0.58 ± 0.08 hrs.), indicating that the subcutaneous absorption of the clinically tested formulation of the compound is slow and rate-limiting.
Distribution: The steady-state volume of distribution (Mean ± SD) following IV administration of Zorbtive® in healthy volunteers is 12.0 ± 1.08 L.
Metabolism: Although the liver plays a role in the metabolism of GH, GH is primarily cleaved in the kidney. GH undergoes glomerular filtration and, after cleavage within the renal cells, the peptides and amino acids are returned to the systemic circulation.
Elimination: The t½ (Mean ± SD) in nine patients with HIV-associated wasting with an average weight of 56.7 ± 6.8 kg, given a fixed dose of 6.0 mg recombinant hGH (r-hGH) subcutaneously was 4.28 ± 2.15 hrs. The renal clearance of r-hGH after subcutaneous administration in nine patients with HIV-associated wasting was 0.0015 ± 0.0037 L/h. No significant accumulation of r-hGH appears to occur after 6 weeks of dosing as indicated.
Special Populations:
Pediatric: Available evidence suggests that r-hGH clearances are similar in adults and children, but no pharmacokinetic studies have been conducted in children with short bowel syndrome.
Gender: Biomedical literature indicates that a gender-related difference in the mean clearance of r-hGH could exist (clearance of r-hGH in males > clearance of r-hGH in females). However, no gender-based analysis is available in normal volunteers or patients with short bowel syndrome.
Race: No data are available.
Renal Insufficiency: It has been reported that individuals with chronic renal failure tend to have decreased r-hGH clearance compared to normals, but there are no data on Zorbtive® use in the presence of renal insufficiency.
Hepatic Insufficiency: A reduction in r-hGH clearance has been noted in patients with severe liver dysfunction. However, the clinical significance of this in short bowel syndrome patients is unknown.
CLINICAL STUDIES
A randomized, double-blind, controlled, parallel-group Phase III clinical study evaluated the efficacy and safety of the administration of Zorbtive® in subjects with Short Bowel Syndrome (SBS) who were dependent on intravenous parenteral nutrition (IPN) for nutritional support. The primary endpoint was the change in weekly total IPN volume defined as the sum of the volumes of IPN, supplemental lipid emulsion (SLE), and intravenous hydration fluid. The secondary endpoints were the change in weekly IPN caloric content and the change in the frequency of IPN administration per week. Subjects received either Zorbtive® placebo with the nutritional supplement, glutamine (n=9), Zorbtive® without glutamine (n=16) or Zorbtive® with glutamine (n=16). All 3 groups received a specialized diet. Following a two-week equilibration period, treatment was administered in a double-blind manner over a further period of four weeks. The dosing of Zorbtive® was approximately 0.1 mg/kg/day for 4 weeks. During the double-blind treatment portion of the trial, the glutamine was given at a daily dose of 30 g. The mean baseline IPN volume, mean IPN caloric content, and mean frequency of IPN administration are provided in Table 1. Mean reductions in IPN volume, IPN caloric content and the frequency of IPN administration in each patient group were significantly greater in both Zorbtive®-treated groups than in the group treated with Zorbtive® placebo. These changes are tabulated in Table 1.
| SOD[GLN] 1 | r-hGH + SOD 1 | r-hGH + SOD[GLN] 1 | |
| 1 SOD[GLN] = Specialized Oral Diet supplemented with Glutamine ; r-hGH + SOD = Human Growth Hormone plus Specialized Oral Diet; r-hGH + SOD[GLN] = Human Growth Hormone plus Specialized Oral Diet supplemented with Glutamine | |||
| * p = 0.043, treatment comparison between r-hGH + SOD versus SOD[GLN] | |||
| ** p <0.001, treatment comparison between r-hGH + SOD[GLN] versus SOD[GLN] | |||
| Total IPN volume (L/wk) | |||
| Mean at Baseline | 13.5 | 10.3 | 10.5 |
| Mean Change | -3.8 | -5.9 | -7.7 |
| Treatment Differences (with GLN) | -2.1* | -3.9** | |
| Total IPN Calories (kcal/wk) | |||
| Mean at Baseline | 8570.4 | 7634.7 | 7895.0 |
| Mean Change | -2633.3 | -4338.3 | -5751.2 |
| Treatment Differences (with GLN) | -1705.0 | -3117.9 | |
| Frequency of IPN or SLE (days/wk) | |||
| Mean at Baseline | 5.9 | 5.1 | 5.4 |
| Mean Change | -2.0 | -3.0 | -4.2 |
| Treatment Differences (with GLN) | -1.0 | -2.2 | |
ZORBTIVE INDICATIONS AND USAGE
Zorbtive® [somatropin (rDNA origin) for injection] is indicated for the treatment of Short Bowel Syndrome in patients receiving specialized nutritional support. Zorbtive® therapy should be used in conjunction with optimal management of Short Bowel Syndrome.
Specialized nutritional support may consist of a high carbohydrate, low-fat diet, adjusted for individual patient requirements and preferences. Nutritional supplements may be added according to the discretion of the treating physician. Optimal management of Short Bowel Syndrome may include dietary adjustments, enteral feedings, parenteral nutrition, fluid and micronutrient supplements, as needed.
ZORBTIVE CONTRAINDICATIONS
Growth hormone therapy should not be initiated in patients with acute critical illness due to complications following open heart or abdominal surgery, multiple accidental trauma or acute respiratory failure. Two placebo-controlled clinical trials in non-growth hormone deficient adult patients (n=522) with these conditions revealed a significant increase in mortality (41.9% vs. 19.3%) among somatropin-treated patients (doses 5.3-8 mg/day) compared to those receiving placebo (See “WARNINGS”).
Zorbtive® is contraindicated in patients with active neoplasia (either newly diagnosed or recurrent). Any anti-tumor therapy should be completed prior to starting therapy with Zorbtive®. Zorbtive® [somatropin (rDNA origin) for injection] reconstituted with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) should not be administered to patients with a known sensitivity to Benzyl Alcohol. (See “WARNINGS”)
Zorbtive® is contraindicated in patients with a known hypersensitivity to growth hormone.
WARNINGS
Benzyl Alcohol as a preservative in Bacteriostatic Water for Injection, USP has been associated with toxicity in newborns. If sensitivity to the diluent occurs, Zorbtive® [somatropin (rDNA origin) for injection] may be reconstituted with Sterile Water for Injection, USP. When Zorbtive® is reconstituted in this manner, the reconstituted solution should be used immediately and any unused portion should be discarded.
See “CONTRAINDICATIONS” for information regarding increased mortality in growth hormone-treated patients with acute critical illnesses in intensive care units due to complications following open heart or abdominal surgery, multiple accidental trauma or acute respiratory failure. The safety of continuing growth hormone treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with growth hormone in patients developing acute critical illnesses should be weighed against the potential risk.
PRECAUTIONS
General: Zorbtive® [somatropin (rDNA origin) for injection] therapy should be carried out under the regular guidance of a physician who is experienced in the diagnosis and management of short bowel syndrome.
Patients should be informed that allergic reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs.
Recombinant human growth hormone (r-hGH) has been associated with acute pancreatitis.
The use of somatropin has been associated with cases of new onset impaired glucose intolerance, new onset type 2 diabetes mellitus and exacerbation of preexisting diabetes mellitus have been reported in patients receiving somatropin. Some patients developed diabetic ketoacidosis and diabetic coma. In some patients, these conditions improved when somatropin was discontinued, while in others the glucose intolerance persisted. Some patients necessitated initiation or adjustment of antidiabetic treatment while on somatropin. Patients with other risk factors for glucose intolerance should be monitored closely during Zorbtive® therapy.
No cases of intracranial hypertension (IH) have been observed among patients with short bowel syndrome treated with Zorbtive®. The syndrome of IH, with papilledema, visual changes, headache, and nausea and/or vomiting has been reported in a small number of children with growth failure treated with growth hormone products. Nevertheless, funduscopic evaluation of patients is recommended at the initiation and periodically during the course of Zorbtive® therapy.
Increased tissue turgor (swelling, particularly in the hands and feet) and musculoskeletal discomfort (pain, swelling and/or stiffness) may occur during treatment with Zorbtive®, but may resolve spontaneously, with analgesic therapy, or after reducing the frequency of dosing (see “DOSAGE AND ADMINISTRATION”).
Carpal tunnel syndrome may occur during treatment with somatropin. If the symptoms of carpal tunnel syndrome do not resolve by decreasing the dose or frequency of somatropin, it is recommended that treatment be discontinued.
Patients being treated with Zorbtive® should be informed of the potential benefits and risks associated with treatment. Patients should be instructed to contact their physician should they experience any side effects or discomfort during treatment with Zorbtive®.
It is recommended that Zorbtive® be administered using sterile, disposable syringes and needles. Patients should be thoroughly instructed in the importance of proper disposal and cautioned against any reuse of needles and syringes. An appropriate container for the disposal of used syringes and needles should be employed.
Patients should be instructed to rotate injection sites to avoid localized tissue atrophy.
Formal drug interaction studies have not been conducted.
Somatropin inhibits 11ß-hydroxysteroid dehydrogenase type 1 (11 ßHSD-1) in adipose/ hepatic tissue and may significantly impact the metabolism of cortisol and cortisone. As a consequence, in patients treated with somatropin, previously undiagnosed primary (and secondary) hypoadrenalism may be unmasked requiring glucocorticoid replacement therapy. In addition, patients treated with glucocorticoid replacement therapy for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of the 11 ßHSD-1 enzyme.
Long-term animal studies for carcinogenicity have not been performed with Zorbtive®. There is no evidence from animal studies to date of Zorbtive®-induced mutagenicity or impairment of fertility.
Pregnancy Category B. Reproduction studies have been performed in rats and rabbits. Doses up to 5 to 10 times the human dose, based on body surface area, have revealed no evidence of impaired fertility or harm to the fetus due to Zorbtive®. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
It is not known whether Zorbtive® is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Zorbtive® is administered to a nursing woman.
There are no formal studies in pediatric patients with short bowel syndrome.
Clinical studies with Zorbtive® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly patients may be more sensitive to growth hormone action, and may be more prone to develop adverse reactions. Thus, dose selection for an elderly patient should be cautious, usually starting at a lower dose.
ZORBTIVE ADVERSE REACTIONS
Table 2 summarizes the number of subjects by system-organ class who experienced an adverse event during the 4-week treatment period of the Phase III SBS study. To be listed in Table 2, an adverse event must have occurred in more than 10% of subjects in any treatment group.
| Adverse Experiences | SOD[GLN]1
n=9 n (%) |
r-hGH+SOD1
n=16 n (%) |
r-hGH+SOD[GLN]1
n=16 n (%) |
| 1 SOD[GLN] = Specialized Oral Diet supplemented with Glutamine; r-hGH + SOD = Human Growth Hormone plus Specialized Oral Diet; r-hGH + SOD[GLN] = Human Growth Hormone plus Specialized Oral Diet supplemented with Glutamine | |||
| Total Number of Subjects with At Least One AE | 8 (89) | 16 (100) | 16 (100) |
| Body as a Whole, General Disorders | 4 (44) | 15 (94) | 15 (94) |
| Edema, Peripheral | 1 (11) | 11 (69) | 13 (81) |
| Edema, Facial | 0 (0) | 8 (50) | 7 (44) |
| Pain | 1 (11) | 3 (19) | 1 (6) |
| Chest Pain | 0 (0) | 3 (19) | 0 (0) |
| Fever | 2 (22) | 0 (0) | 1 (6) |
| Back Pain | 1 (11) | 1 (6) | 0 (0) |
| Flu-like Disorder | 1 (11) | 0 (0) | 1 (6) |
| Malaise | 0 (0) | 2 (13) | 0 (0) |
| Edema, Generalized | 0 (0) | 2 (13) | 0 (0) |
| Abdomen Enlarged | 1 (11) | 0 (0) | 0 (0) |
| Allergic Reaction | 1 (11) | 0 (0) | 0 (0) |
| Rigors (Chills) | 1 (11) | 0 (0) | 0 (0) |
| Gastrointestinal System Disorders | 6 (67) | 12 (75) | 12 (75) |
| Flatulence | 2 (22) | 4 (25) | 4 (25) |
| Abdominal Pain | 1 (11) | 4 (25) | 2 (13) |
| Nausea | 0 (0) | 2 (13) | 5 (31) |
| Tenesmus | 3 (33) | 1 (6) | 3 (19) |
| Vomiting | 1 (11) | 3 (19) | 3 (19) |
| Hemorrhoids | 1 (11) | 1 (6) | 0 (0) |
| Mouth Dry | 1(11) | 1 (6) | 0 (0) |
| Musculoskeletal System Disorders | 1 (11) | 7 (44) | 7 (44) |
| Arthralgia | 0 (0) | 7 (44) | 5 (31) |
| Myalgia | 1 (11) | 2 (13) | 0 (0) |
| Resistance Mechanism Disorders | 4 (44) | 6 (38) | 3 (19) |
| Infection | 3 (33) | 0 (0) | 1 (6) |
| Infection Bacterial | 1 (11) | 3 (19) | 0 (0) |
| Infection Viral | 0 (0) | 1 (6) | 2 (13) |
| Moniliasis | 0 (0) | 2 (13) | 0 (0) |
| Application Site Disorders | 1 (11) | 5 (31) | 4 (25) |
| Injection Site Reaction | 1 (11) | 3 (19) | 4 (25) |
| Injection Site Pain | 5 (31) | 0 (0) | 0 (0) |
| Central and Peripheral Nervous System Disorders | 2 (22) | 4 (25) | 4 (25) |
| Dizziness | 0 (0) | 1 (6) | 2 (13) |
| Headache | 1 (11) | 1 (6) | 1 (6) |
| Hypoasthesia | 1 (11) | 1 (6) | 1 (6) |
| Skin and Appendages Disorders | 2 (22) | 4 (25) | 4 (25) |
| Rash | 0 (0) | 1 (6) | 2 (13) |
| Pruritis | 1 (11) | 0 (0) | 1 (6) |
| Sweating Increased | 0 (0) | 2 (13) | 0 (0) |
| Nail Disorder | 1 (11) | 0 (0) | 0 (0) |
| Respiratory System Disorders | 1 (11) | 1 (6) | 5 (31) |
| Rhinitis | 1 (11) | 0 (0) | 3 (19) |
| Metabolic and Nutritional Disorders | 1 (11) | 3 (19) | 1 (6) |
| Dehydration | 1 (11) | 3 (19) | 0 (0) |
| Thirst | 1 (11) | 0 (0) | 0 (0) |
| Urinary System Disorders | 1 (11) | 2 (13) | 1 (6) |
| Pyelonephritis | 1 (11) | 0 (0) | 0 (0) |
| Psychiatric Disorders | 2 (22) | 1 (6) | 0 (0) |
| Depression | 2 (22) | 0 (0) | 0 (0) |
| Reproductive Disorders, Female | 1 (11) | 2 (13) | 0 (0) |
| Breast Pain Female | 1 (11) | 1 (6) | 0 (0) |
| Hearing and Vestibular Disorders | 0 (0) | 0 (0) | 2 (13) |
| Ear or Hearing Symptoms | 0 (0) | 0 (0) | 2 (13) |
Table 3 summarizes the number of subjects by system-organ class who experienced an adverse event during the 12-week follow-up period of the Phase III SBS study. To be listed in Table 3, an adverse event must have occurred in more than 10% of subjects in any treatment group.
| Adverse Experiences | SOD[GLN]1
n=9 n (%) |
r-hGH+SOD1
n=15 n (%) |
r-hGH+SOD[GLN]1
n=16 n (%) |
| 1 SOD[GLN] = Specialized Oral Diet supplemented with Glutamine ;r-hGH + SOD = Human Growth Hormone plus Specialized Oral Diet; r-hGH + SOD[GLN] = Human Growth Hormone plus Specialized Oral Diet supplemented with Glutamine | |||
| Total Number of Subjects with At Least One AE | 7 (78) | 12 (80) | 13 (81) |
| Gastrointestinal System Disorders | 3 (33) | 7 (47) | 7 (44) |
| Nausea | 2 (22) | 3 (20) | 0 (0) |
| Vomiting | 0 (0) | 2 (13) | 3 (19) |
| Abdominal Pain | 0 (0) | 3 (20) | 1 (6) |
| Tenesmus | 1 (11) | 0 (0) | 3 (19) |
| Pancreatitis | 1 (11) | 0 (0) | 1 (6) |
| Constipation | 1 (11) | 0 (0) | 0 (0) |
| Crohn's Disease Aggravated | 1 (11) | 0 (0) | 0 (0) |
| Gastric Ulcer | 1 (11) | 0 (0) | 0 (0) |
| Gastrointestinal Fistula | 1 (11) | 0 (0) | 0 (0) |
| Resistance Mechanism Disorders | 5 (56) | 6 (40) | 5 (31) |
| Infection Bacterial | 3 (33) | 0 (0) | 2 (13) |
| Infection Viral | 1 (11) | 3 (20) | 1 (6) |
| Infection | 1 (11) | 1 (7) | 2 (13) |
| Sepsis | 0 (0) | 3 (20) | 1 (6) |
| Body as a Whole, General Disorders | 1 (11) | 4 (27) | 2 (13) |
| Fever | 1 (11) | 2 (13) | 1 (6) |
| Fatigue | 0 (0) | 2 (13) | 0 (0) |
| Respiratory System Disorders | 1 (11) | 2 (13) | 4 (25) |
| Rhinitis | 0 (0) | 1 (7) | 3 (19) |
| Laryngitis | 1 (11) | 0 (0) | 0 (0) |
| Pharyngitis | 1 (11) | 0 (0) | 0 (0) |
| Reproductive Disorders, Female | 1 (11) | 0 (0) | 4 (25) |
| Vaginal Fungal Infection | 1 (11) | 0 (0) | 0 (0) |
| Skin and Appendages Disorders | 1 (11) | 2 (13) | 2 (13) |
| Rash | 1 (11) | 1 (7) | 0 (0) |
| Musculoskeletal System Disorders | 0 (0) | 2 (13) | 2 (13) |
| Arthralgia | 0 (0) | 2 (13) | 2 (13) |
| Psychiatric Disorders | 1 (11) | 0 (0) | 1 (6) |
| Depression | 1 (11) | 0 (0) | 0 (0) |
| Insomnia | 1 (11) | 0 (0) | 0 (0) |
| Urinary System Disorders | 2 (22) | 0 (0) | 0 (0) |
| Pyelonephritis | 1 (11) | 0 (0) | 0 (0) |
| Renal Calculus | 1 (11) | 0 (0) | 0 (0) |
| Application Site Disorders | 1 (11) | 0 (0) | 0 (0) |
| Injection Site Reaction | 1 (11) | 0 (0) | 0 (0) |
| Liver and Biliary System Disorders | 1 (11) | 0 (0) | 0 (0) |
| Hepatic Function Abnormal | 1 (11) | 0 (0) | 0 (0) |
| Vascular Extracardiac Disorders | 1 (11) | 0 (0) | 0 (0) |
| Vascular Disorder | 1 (11) | 0 (0) | 0 (0) |
Adverse events that occurred in 1% to less than 10% of study participants receiving Zorbtive® in the placebo-controlled clinical efficacy trial are listed below by body system. The list of adverse events has been compiled regardless of casual relationship to Zorbtive®.
Body as a Whole, General: edema, periorbital edema
Gastrointestinal System: melena, rectal hemorrhage, mouth disorder, steatorrhea
Musculoskeletal System: arthritis, arthropathy, bursitis, cramps
Resistance Mechanism Disorders: fungal infection
Application Site Disorders: reaction pain, inflammation at injection sites
Central and Peripheral Nervous System: parasthesia, phantom pain, visual field defect
Respiratory System: bronchospasm, dyspnea, pharyngitis, respiratory disorder, respiratory infection
Platelet, Bleeding and Clotting Disorders: purpura, prothrombin decrease
Skin and Appendages: skin disorder, increased sweating, alopecia, bullous eruption
Psychiatric: insomnia
Metabolic and Nutritional: hypomagnesemia,
Urinary System Disorders: dysuria, urinary tract infection, abnormal urine
Reproduction Disorders, Female: breast enlargement, vaginal fungal infection
Heart Rate and Rhythm Disorders: tachycardia
Vascular Extracardiac Disorders: vasodilatation
The safety profile of patients receiving Zorbtive® with glutamine was similar to the safety profile of patients receiving Zorbtive® without glutamine. During the baseline period, 88% of patients receiving Zorbtive® with glutamine, 88% of patients receiving Zorbtive® without glutamine, and 78% of patients receiving Zorbtive® placebo with glutamine reported baseline signs and symptoms (BSS). During the treatment period, 100% of patients receiving Zorbtive® with and without glutamine reported at least one adverse event (AE), whereas 89% of patients receiving Zorbtive® placebo with glutamine reported at least one AE. During the follow-up period, 81% of patients receiving Zorbtive® with glutamine, 80% of patients receiving Zorbtive® without glutamine and 78% of patients receiving Zorbtive® placebo with glutamine experienced at least one AE. Comparison of the number of serious adverse events (SAEs) before and during treatment demonstrates that this subject population experiences numerous BSSs and AEs due to their underlying conditions and parenteral nutrition complications. Four subjects (25%) receiving Zorbtive® without glutamine and one subject (11%) receiving Zorbtive® placebo with glutamine experienced at least one SAE during the treatment period (Zorbtive® without glutamine: chest pain, purpura, fungal infection, pharyngitis; Zorbtive® placebo with glutamine: hemorrhoids). None of the subjects receiving Zorbtive® with glutamine experienced SAEs during the treatment period. During the follow-up period, 3 subjects (19%) receiving Zorbtive® with glutamine, 5 subjects (33%) receiving Zorbtive® without glutamine and 3 subjects (33%) receiving Zorbtive® placebo with glutamine experienced at least one SAE. There were no deaths in this study.
OVERDOSAGE
Glucose intolerance can occur with overdosage. Long-term overdosage with growth hormone could result in signs and symptoms of acromegaly.
ZORBTIVE DOSAGE AND ADMINISTRATION
Zorbtive® should be administered to patients with short bowel syndrome (SBS) at a dose of approximately 0.1 mg/kg subcutaneously daily to a maximum of 8 mg daily. Administration for more than 4 weeks has not been adequately studied.
Injections should be administered daily for 4 weeks. Changes to concomitant medications should be avoided. Patients and physicians should monitor for adverse events. Treat moderate fluid retention and arthralgias symptomatically or dose reduce by 50%. Discontinue Zorbtive® for up to 5 days for severe toxicities. Upon resolution of symptoms, resume at 50% of original dose. Permanently discontinue treatment if severe toxicity recurs or does not disappear within 5 days.
Injection sites should be rotated.
Safety and effectiveness in pediatric patients with short bowel syndrome have not been established.
Each vial of Zorbtive® 8.8 mg is reconstituted in 1 to 2 mL of Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol). See Table 4 below for expected concentration after reconstitution. Approximately 10% mechanical loss can be associated with reconstitution and administration from multi-dose vials. For patients sensitive to Benzyl Alcohol, see “WARNINGS”.
| 1 mL | 2 mL | |
| 8.8 mg | 8.8 | 4.4 |
To reconstitute Zorbtive®, inject the diluent into the vial of Zorbtive® aiming the liquid against the glass vial wall. Swirl the vial with a gentle rotary motion until contents are dissolved completely. The Zorbtive® solution should be clear immediately after reconstitution. DO NOT INJECT Zorbtive® if the reconstituted product is cloudy immediately after reconstitution or after refrigeration. The reconstituted Zorbtive® 8.8 mg can be refrigerated (2-8oC/36-46oF) for up to 14 days. Occasionally, after refrigeration, small colorless particles may be present in the Zorbtive® 8.8 mg solution. This is not unusual for proteins like Zorbtive®. Allow refrigerated solution to come to room temperature prior to administration. A standard insulin-type subcutaneous syringe is recommended for administration.
STABILITY AND STORAGE
Before Reconstitution: Vials of Zorbtive® and diluent should be stored at room temperature, (15-30°C/59-86°F). Expiration dates are stated on product labels.
After Reconstitution with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol. The reconstituted solution should be stored under refrigeration (2-8oC/36-46oF) for up to 14 days. Avoid freezing reconstituted vials of Zorbtive®.
Zorbtive® [somatropin (rDNA origin) for injection] is available in the following form:
Zorbtive® vial containing 8.8 mg somatropin (mammalian-cell) with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol). Package of 7 vials.
NDC 44087-3388-7
Manufactured for: EMD Serono, Inc., Rockland, MA 02370
January 2012
Patient Package Insert
Patient Information
Zorbtive® (Zorb-tiv)
somatropin (so-ma-tro-pin)
Read the Patient Information and the Instructions for Injection that comes with Zorbtive® before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor or healthcare professional about your medical condition or treatment. If you do not understand the information, or have any questions about Zorbtive®, talk with your doctor or pharmacist.
What is Zorbtive®?
Zorbtive® is a man-made human growth hormone (hGH). Zorbtive® is similar to the growth hormone your body makes. Zorbtive® is used to treat Short Bowel Syndrome (SBS) in patients who are on a specialized diet. When used with a special diet, Zorbtive® may help your bowel take in more water, electrolytes and nutrients and lower your need for I.V. (intravenous) feedings.
Zorbtive® will not cure Short Bowel Syndrome (SBS).
You will need careful medical follow-up and monitoring of your condition while taking Zorbtive®.
Only doctors who treat SBS should prescribe Zorbtive® therapy.
Who should not take Zorbtive®?
Do not take Zorbtive® if you:
-
Have a serious medical condition after having:
- Open heart surgery
- Stomach area (abdominal) surgery
- An accident
- Breathing problems (respiratory failure)
- Have cancer and are getting treatment for it
- Are allergic to Zorbtive® or to other growth hormone medicines
The active ingredient in Zorbtive® is somatropin. The inactive ingredients are sucrose and phosphoric acid. Benzyl alcohol is a preservative in the Bacteriostatic Water for Injection (water for mixing the multi-dose vial of Zorbtive®). Ask your doctor or pharmacist for plain sterile water for mixing with Zorbtive® if you are allergic to benzyl alcohol. Benzyl alcohol can also harm babies.
Zorbtive® has not been studied in children under 18 years or in adults over 65 years with SBS.
Before taking Zorbtive®, tell your doctor
-
About all your medical conditions, including if you:
- Have diabetes or blood sugar problems. You will need to be monitored carefully while taking Zorbtive®
- Have high blood pressure
- Have pancreas problems
- Have or had cancer
- Have kidney problems
- Have liver problems
- Have carpal tunnel syndrome (a problem that causes numbness and tingling in your wrist and hand)
- Are pregnant or planning to become pregnant. It is not known if Zorbtive® may harm your unborn baby.
- Are breastfeeding. It is not known if Zorbtive® passes into your milk and if it can harm your baby. Talk to your doctor about the best way to feed your baby while taking Zorbtive®.
- About all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. It is not known if Zorbtive® and other medicines may affect each other. Know the medicines you take. Keep a list of all your medicines to show to your doctor and pharmacist. Especially tell your doctor if you take medicines for diabetes. The doses of your diabetes medicines may need adjustment if you take Zorbtive®. Tell your doctor if you take other growth hormone medicines.
How should I take Zorbtive®?
See the end of this leaflet for detailed Instructions for Injecting Zorbtive®.
- Take Zorbtive® exactly as prescribed by your doctor. Zorbtive® is given by injection under the skin (subcutaneous) every day for four weeks (28 days). Your doctor will tell you how much Zorbtive® to use, and may change the dose based on how your body responds. Do not change your dose of Zorbtive® unless told to by your doctor. You must also follow the special diet that your doctor prescribes while taking Zorbtive®. You will be told the amount of food to eat and the times of day that you should eat. Your doctor may adjust your special diet during treatment with Zorbtive®. Do not change your special diet unless you talk with your doctor.
- Do not inject Zorbtive® unless your doctor has taught you the right way and you understand everything.
- Change your injection site each time Zorbtive® is injected. This will lower the chance of getting a skin reaction at the spot where you inject Zorbtive®. Do not inject Zorbtive® into an area of skin that is sore, red, infected or damaged.
- Always use a new, unopened, needle and syringe for each injection. Never reuse needles and syringes. Discard used needles and syringes in a puncture resistant container called a Sharps container, which you can get from your doctor or pharmacist.
- If you miss a dose of Zorbtive®, take it as soon as you remember or are able to take it. If you miss more than 3 consecutive doses, call your doctor for further instructions.
- Do not take more than one dose each day. If you take too much Zorbtive®, call your doctor right away.
- You should have careful monitoring of your condition while taking Zorbtive®. Your doctor may do blood tests and eye tests before you start Zorbtive® and regularly while you are taking it.
- Treatment with Zorbtive® may stop if you are put in the hospital for a serious illness, injury or surgery.
What should I avoid while taking Zorbtive®?
- You should avoid:
- Starting any other new medicines unless prescribed by your doctor.
- Making changes to the other medicines you take unless prescribed by your doctor.
- Drinking water, soda, fruit juices. You must be careful about the liquids you drink. Your doctor will tell you which liquids are okay to drink.
- Drinking alcoholic beverages. Irritation of your stomach may result after drinking alcoholic beverages and may make your SBS worse. Also, alcoholic beverages may interact with medications you are already taking.
What kind of food should I eat during my treatment with Zorbtive®?
- Your doctor will prescribe for you the types and amounts of foods you should eat during your treatment with Zorbtive®. These foods are not special and can be purchased from your local market. Your likes and dislikes will also be taken into consideration when your eating plan is created.
- Your eating plan will consist of carbohydrates (about 50-55% of your total food for the day), protein (about 20% of your food for the day) and fat (about 25-30% of your food for the day). Good carbohydrates for you to eat are rice, potatoes, pasta, cereal and grain products; simple sugars should not be eaten very often. Good proteins for you to eat are chicken, fish and turkey; high fatty meats like steak and pork should be avoided. Fats such as soybean oil and safflower oil are good to include in your meals.
- Your doctor will advise you on how many times a day you should eat; often more smaller meals throughout the day are better than 2-3 large meals. Your diet will be adjusted during your treatment with Zorbtive® as your doctor finds necessary. It is important that you keep to the eating plan your doctor gives to you.
What are the possible side effects of Zorbtive®?
Zorbtive® can cause serious side effects including:
- Allergic reactions including trouble breathing, hives and skin that becomes red and warm all over the body. Stop Zorbtive® and get emergency medical help right away if you get any of these symptoms.
- Pancreatitis. The pancreas is a large gland that is behind the stomach. Zorbtive® can cause sudden pancreatitis attacks. Symptoms of sudden pancreatitis include severe pain in the upper stomach area, swollen and tender stomach area, sweating, nausea, vomiting, fever, skin and whites of the eyes turn yellow, and a fast heart beat.
- Diabetes or blood sugar problems. Zorbtive® can cause new diabetes or blood sugar problems that do not go away when Zorbtive® is stopped. Zorbtive® can worsen the diabetes and blood sugar problems you already have. Your diabetes medicines may need to be adjusted while you are taking Zorbtive®.
- High blood pressure in your brain (intracranial hypertension or IH). IH can cause headaches, vision changes, swelling of the eye nerve, nausea and vomiting. Your doctor may check your eyes before and during treatment with Zorbtive®.
- Muscle or joint pain, swelling or stiffness. Do not take any pain medicine unless your doctor tells you to.
- Carpal tunnel syndrome (a problem that causes numbness and tingling in your wrist and hand)
Call your doctor right away if you get any of the following symptoms while taking Zorbtive®:
- Tingling feeling in your hands or wrists
- Increased thirst, increased urination or dizziness
- Swelling of the head, face, hands or feet
- Unusual headaches, problems with eyesight, nausea and vomiting
Zorbtive® may also cause nausea, vomiting, stomach pain and gas. These symptoms may be caused by your SBS or by your treatment with Zorbtive®. Talk to your doctor if you get these symptoms. Your doctor may prescribe medicine to help with these symptoms. It is important for you to follow your special meal plan.
Reactions at the injection site. Soreness, redness, rash, skin bumps, itching, pain, bruising or swelling may happen at the place of injection. Changing your skin injection site each time may lower the chances for these side effects.
These are not all the side effects of Zorbtive®. For more information, ask your doctor or pharmacist.
How Do I Store Zorbtive®?
- Store unmixed vials of Zorbtive® and sterile water at room temperature 59° to 86°F (15° to 30°C)
- If mixing the medicine with plain Sterile Water for Injection, USP, mix and use it right away and discard any unused medicine.
- If mixing the medicine with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol), the mixed medicine can be stored refrigerated at 36o to 46oF (2o to 8oC) for up to 14 days (2 weeks). After refrigeration, small particles may be present. This is not unusual for proteins like Zorbtive®. Do not freeze the mixed medicine. Allow the medicine to warm to room temperature before using.
- Safely throw away medicine that is out-of-date or no longer needed. The expiration date is printed on the vial label and the carton.
Keep Zorbtive® and all medicines out of the reach of children.
General Information About Zorbtive®
Medicines are sometimes prescribed for conditions that are not mentioned in the patient information leaflets. Do not use Zorbtive® for a condition for which it was not prescribed. Do not give Zorbtive® to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Zorbtive®. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Zorbtive® that is written for health professionals.
Instructions for Preparing and Giving Yourself an Injection of Zorbtive®
This section explains how to give yourself a Zorbtive® injection. Zorbtive® injections can be fairly easily given because Zorbtive® is injected under the skin (subcutaneously). You may already be taking other medications this way.
WARNING: DO NOT self-administer until you receive proper injection training from your health care professional.
Zorbtive® Vials and Diluent
Zorbtive® multiple-dose packaging includes seven vials of Zorbtive®, 8.8 mg strength, and seven vials of sterile water preserved with benzyl alcohol, labeled ‘Bacteriostatic Water for Injection.’ Patients allergic to benzyl alcohol should not use this bacteriostatic water and should inform their doctor. Benzyl alcohol can also be harmful to newborns.
Supplies Needed for Taking Zorbtive®
Along with the Zorbtive® vials and diluent, your prescription included two kinds of syringes. A 3 cc syringe, with a large, 1-inch needle, is used for mixing the medication. The second, 1 cc syringe, has a small 29-gauge needle, and it is used for injection. Before you take the medication, you will also need cotton balls, alcohol swabs and a sharps disposal container.
You will take Zorbtive® daily. Always start with new, unopened sterile syringes and needles. Never reuse syringes and needles.
Instructions for Preparing and Giving Yourself an Injection of Zorbtive® [somatropin (rDNA origin) for injection]
I. PREPARATION
• Step 1. Gather Your Zorbtive® Injection Supplies.
• Step 2. Clean the Work Surface.
• Step 3. Wash Your Hands.
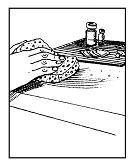
Use soap and water. If someone else is giving the injection, they should put on a pair of disposable gloves now.
II. RECONSTITUTION
• Step 4. Remove Flip-Tops from Drug and Sterile Water Vials.
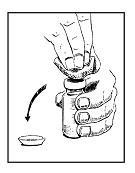
Throw the tops away. Clean both rubber stoppers with a fresh alcohol swab. Do not touch the vial tops with your hands or gloves.
• Step 5. Prepare Larger Needle and Syringe.
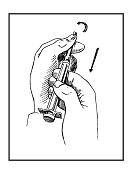
Unwrap the larger needle and syringe, used for mixing the diluent with Zorbtive®. Pull back the plunger to the amount of diluent designated by your doctor. Do not touch the exposed plunger. Remove the plastic tip protector from the needle.
• Step 6. Fill the Syringe with Sterile Water.
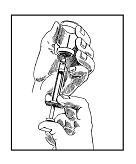
Place the vial of sterile water on your clean work surface. Push the needle straight down through the middle of the rubber stopper on top of the vial. Push the syringe plunger all the way down; this will push air into the vial. You will feel a little pressure.
While holding the plunger down, pick up the vial with your other hand, and turn it upside down. Now, slide the needle tip below the level of water in the vial. Release the plunger. The syringe barrel fills up with water by itself. You can help it along by pulling back on the plunger until it reaches the desired mark on the syringe barrel.
If air bubbles get in, tap the syringe barrel with your fingertips, then gently push the plunger in to move the air bubbles back into the vial. When you have the desired amount of water in the syringe and no air bubbles, wiggle the vial off the needle. Be careful not to touch the needle tip. Do not put the syringe down.
Now, you are ready to mix the sterile water with the drug in the Zorbtive® vial. At this point, you should be holding the needle and syringe containing sterile water without any air bubbles.
• Step 7. Slowly Inject the Sterile Water Against the Inner Wall of the Zorbtive® Vial.
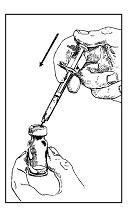
With the vial of Zorbtive® on your clean surface, push the needle, at a slight angle through the rubber stopper, all the way into the top of the Zorbtive® vial. The needle tip should rest against the vial wall. Push the syringe plunger gently and slowly, allowing the water to flow down the side of the vial. Avoid squirting the water directly onto the pellet, which will make the drug solution foamy and hard to use.
• Step 8. Gently Swirl Zorbtive® Vial to Mix.
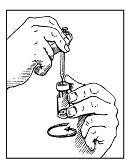
Keep the needle in the vial, steadying it with one hand. Hold the drug vial in your other hand. Move the drug vial in slow, gentle circles to mix the water and the drug together. Do not shake or turn the vial upside down.
Look inside the drug vial. You should see a clear liquid. If you see any powder, keep moving the vial in circles until it disappears.
Note: If you shook the vial too much and the liquid is foamy, let it sit for a few minutes until the bubbles disappear.
Remove the needle and syringe from vial and discard in your sharps disposal container.
III. PREPARING THE ZORBTIVE® INJECTION
• Step 9. Using the Smaller Needle and Syringe, Draw Up Prescribed Dose.
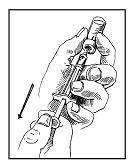
Insert the smaller needle and syringe into the Zorbtive® vial. Turn the vial upside down. Make sure the needle is below the fluid level. Pull back on the plunger to fill the syringe barrel with the designated dose as specified by your physician. DO NOT PULL THE PLUNGER OUT OF THE BARREL. Wiggle the vial off the needle. Do not touch the needle tip with your hands or gloves. Keep the syringe in your hand.
• Step 10. Remove Air Bubbles.

Hold the syringe straight up and down with the needle toward the ceiling. Look at the liquid in the syringe to see if there are any air bubbles. Tap the side of the syringe with your fingertips to loosen any stuck air bubbles. They will float to the top of the liquid.
Push the plunger gently to remove any air in the syringe barrel and needle. Recap the needle. Carefully lay the syringe on a flat, clean surface. Do not touch the needle or allow the needle to touch any surface.
IV. GIVING YOURSELF THE INJECTION
• Step 11. Pick an Injection Site.
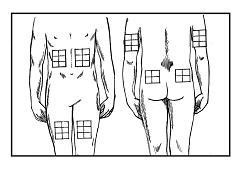
Find an area on your body that makes it easy for you to give the injection. The best sites for giving an injection are those areas with a layer of fat between the skin and muscle, like your thigh or upper arm.
Do not inject in the areas near your navel or waistline. If you are very thin, the outer thigh or upper arm may be best. Do not inject Zorbtive® into an area on your body where the skin is irritated or abnormal in any way.
Most people find the top of the thigh works best. Change the injection location each day to minimize discomfort.
Take an alcohol swab and carefully clean a four-inch square area of skin where you plan to inject. Let the skin dry by itself.
• Step 12. Pinch Skin and Inject Zorbtive®.

Take the cap off the needle. Pinch up a three-inch fold of just-cleaned skin, using your thumb and index finger. A three-inch fold is about as wide as four of your fingers placed together.
Hold the syringe like a pencil and swiftly slide the needle, bevel side up, just under the skin at a slight angle.
After the needle is in, release your hold on the pinched skin and inject the drug using a slow, steady push on the plunger until all the medication is injected and the syringe is empty. After you’ve emptied the syringe, pull the needle straight out of the skin.
Apply gentle pressure with a dry cotton ball or sterile gauze. Applying a cold compress or ice pack to the injection site may help reduce skin reactions. Put a small adhesive bandage strip over the injection site, if desired.
• Step 13. Clean Up and Put Away Supplies.

Do not recap the injection needle. Discard the whole syringe and needle into the sharps disposal container, along with any used gauze patches, sterile wipes and gloves.
After two hours, check the injection site for redness, swelling, or tenderness. If you have a skin reaction that does not clear up in a few days, contact your doctor or nurse.
Always keep your sharps disposal container out of the reach of children. DO NOT throw the needle and syringe in the household trash or recycle.
Save any unused portion of the medication remaining in the vial and store in the refrigerator until your next dose. On the next dose, reconstitute a new vial of Zorbtive®. Then, draw up the remaining medication from the refrigerated vial, emptying the vial. Draw up the balance of prescribed dose from the new vial, until dose amount is reached.
For further information or instruction assistance, you may call the AXIS® Center at 1-877-714-AXIS (2947).
For further information, please refer to Zorbtive® Patient Handbook .
This Patient Package Insert has been approved by the U.S. Food and Drug Administration.
Manufactured for:
EMD Serono, Inc.
Rockland, MA 02370
January 2012
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL



Zorbtivesomatropin (rDNA origin) KIT
| ||||||||||||||||||||||||||||||||||||||||