Zelapar
Valeant Pharmaceuticals International
ZELAPAR (selegiline hydrochloride) Orally Disintegrating Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- ZELAPAR DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- ZELAPAR INDICATIONS AND USAGE
- ZELAPAR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Melanoma
- General
- Phenylketonurics
- Irritation of the Buccal Mucosa
- Dyskinesia
- Effect on Renal Function
- Renally-Impaired Patients
- Hepatically-Impaired Patients
- Withdrawal-Emergent Hyperpyrexia and Confusion
- Hallucinations
- Information for Patients
- Laboratory Tests
- Drug Interactions
- CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- ZELAPAR ADVERSE REACTIONS
- Postmarketing Reports
- OVERDOSAGE
- ZELAPAR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 6 pouches, 10 Tablet Carton
FULL PRESCRIBING INFORMATION
ZELAPAR DESCRIPTION
ZELAPAR ® Orally Disintegrating Tablets contain selegiline hydrochloride, a levorotatory acetylenic derivative of phenthylamine. Selegiline hydrochloride is described chemically as (-)-(R)-N,α-dimethyl-N-2-propynylphenethylamine hydrochloride and its structural formula is:
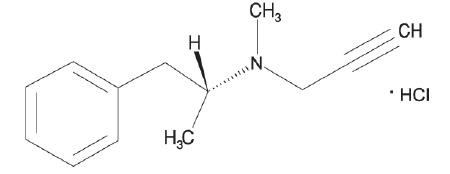
Its empirical formula is C13H17N• HCl, representing a molecular weight of 223.75. Selegiline hydrochloride is a white to almost white crystalline powder that is freely soluble in water, chloroform, and methanol.
ZELAPAR® Orally Disintegrating Tablets are available for oral administration (not to be swallowed) in a strength of 1.25 mg. Each lyophilized orally disintegrating tablet contains the following inactive ingredients: gelatin, mannitol, glycine, aspartame, citric acid, yellow iron oxide, and grapefruit flavor.
CLINICAL PHARMACOLOGY
The mechanisms accounting for selegiline's beneficial adjunctive action in the treatment of Parkinson's disease are not fully understood. Inhibition of monoamine oxidase type B (MAO-B) activity is generally considered to be of primary importance; in addition, there is evidence that selegiline may act through other mechanisms to increase dopaminergic activity.
Selegiline is best known as an irreversible inhibitor of monoamine oxidase (MAO), an intracellular enzyme associated with the outer membrane of mitochondria. Selegiline inhibits MAO by acting as a suicide substrate for the enzyme; that is, converted by MAO to an active moiety which combines irreversibly with the active site and/or the enzyme's essential flavin adenine dinucleotide (FAD) cofactor. Because selegiline has greater affinity for type B rather than for type A active sites, it can serve as a selective inhibitor of MAO type B if it is administered at the recommended dose. However, even for "selective" MAO-B inhibitors, the selectivity for inhibiting MAO-B typically diminishes and is ultimately lost as the dose is increased beyond particular dose levels.
MAOs are widely distributed throughout the body; their concentration is especially high in liver, kidney, stomach, intestinal wall, and brain. MAOs are currently subclassified into two types, A and B, which differ in their substrate specificity and tissue distribution. In humans, intestinal MAO is predominantly type A (MAO-A), while most of that in brain is type B (MAO-B).
In CNS neurons, MAO plays an important role in the catabolism of catecholamines (dopamine, norepinephrine and epinephrine) and serotonin. MAOs are also important in the catabolism of various exogenous amines found in a variety of foods and drugs. MAO in the GI tract and liver (primarily type A), for example, is thought to provide vital protection from exogenous amines (e.g., tyramine) that have the capacity, if absorbed intact, to cause a hypertensive crisis, the so-called cheese reaction. (If large amounts of certain exogenous amines gain access to the systemic circulation - e.g., from fermented cheese, red wine, herring, over-the-counter cough/cold medications, etc. - they are taken up by adrenergic neurons and displace norepinephrine from storage sites within membrane bound vesicles. Subsequent release of the displaced norepinephrine causes the rise in systemic blood pressure, etc.)
In theory, since MAO-A of the gut is not inhibited, patients treated with ZELAPAR® at the recommended dose of 2.5 mg a day should be able to take medications containing pharmacologically active amines and consume tyramine-containing foods without risk of uncontrolled hypertension.
Although rare, a few reports of hypertensive reactions have occurred in patients receiving swallowed selegiline at the recommended dose (a dose believed to be selective for MAO-B), with tyramine-containing foods. In addition, one case of hypertensive crisis has been reported in a patient taking the recommended dose of swallowed selegiline and a sympathomimetic medication (ephedrine). The pathophysiology of the cheese reaction is complicated and, in addition to its ability to inhibit MAO-B selectively, selegiline's relative freedom from this reaction has been attributed to an ability to prevent tyramine and other indirect acting sympathomimetics from displacing norepinephrine from adrenergic neurons. However, until the pathophysiology of the cheese reaction is more completely understood, it seems prudent to assume that ZELAPAR® can ordinarily only be used safely without dietary restrictions at doses where it presumably selectively inhibits MAO-B (e.g., 2.5 mg/day). Safe use of ZELAPAR® at doses above 2.5 mg daily without dietary tyramine restrictions has not been established.
In short, attention to the dose-dependent nature of ZELAPAR®'s selectivity is critical if it is to be used without elaborate restrictions being placed on diet and concomitant drug use. Physicians and patients should be mindful that, as noted above, a few cases of hypertensive crisis have been reported with the swallowed use of selegiline, even at its recommended dose. (See WARNINGS and PRECAUTIONS.)
Because selegiline's inhibition of MAO-B is irreversible, it is impossible to predict the extent of MAO-B inhibition from steady state plasma levels. For the same reason, it is not possible to predict the rate of recovery of MAO-B activity as a function of plasma levels. The recovery of MAO-B activity is a function of de novo protein synthesis; however, information about the rate of de novo protein synthesis is not yet available. Although platelet MAO-B activity returns to the normal range within 5 to 7 days of selegiline discontinuation, the linkage between platelet and brain MAO-B inhibition is not fully understood nor is the relationship of MAO-B inhibition to the clinical effect established.
It is important to be aware that selegiline may have pharmacological effects unrelated to MAO-B inhibition. As noted above, there is some evidence that it may increase dopaminergic activity by other mechanisms, including interfering with dopamine re-uptake at the synapse. Effects resulting from swallowed selegiline may also be mediated through its metabolites. However, the extent to which these metabolites contribute to the effects of swallowed selegiline are unknown. Since ZELAPAR® is primarily absorbed across the buccal mucosa, thereby bypassing the significant first pass metabolism seen with swallowed selegiline, the concentrations of these metabolites (including amphetamine and methamphetamine) are negligible.
Rationale for the Use of Selective Monoamine Oxidase Type B Inhibitor in Parkinson's Disease
Many of the prominent symptoms of Parkinson's disease are due to a deficiency of striatal dopamine that is the consequence of a progressive degeneration and loss of a population of dopaminergic neurons which originate in the substantia nigra of the midbrain and project to the basal ganglia or striatum. Early in the course of Parkinson's disease, the deficit in the capacity of these neurons to synthesize dopamine can be overcome by administration of exogenous levodopa, usually given in combination with a peripheral decarboxylase inhibitor (carbidopa).
With the passage of time, due to the progression of the disease and/or the effect of sustained treatment, the efficacy and quality of the therapeutic response to levodopa diminishes. Thus, after several years of levodopa treatment, the response, for a given dose of levodopa, is shorter, has less predictable onset and offset (i.e., there is wearing "OFF"), and is often accompanied by side effects (e.g., dyskinesia, akinesias, "ON"-"OFF" phenomena, freezing, etc.).
This deteriorating response is currently interpreted as a manifestation of the inability of the ever-decreasing population of intact nigrostriatal neurons to synthesize and release adequate amounts of dopamine.
MAO-B inhibition may be useful in this setting because, by blocking the catabolism of dopamine, it would increase the net amount of dopamine available (i.e., it would increase the pool of dopamine). Whether or not this mechanism or an alternative one actually accounts for the observed beneficial effects of adjunctive selegiline is unknown.
ZELAPAR®'s benefit in Parkinson's disease has only been documented as an adjunct to levodopa/carbidopa in patients with significant "OFF" periods. It is important to note that attempts to treat Parkinsonian patients with combinations of levodopa and currently marketed non-selective MAO inhibitors were abandoned because of multiple side effects including hypertension, increase in involuntary movement, and toxic delirium.
PHARMACOKINETICS
Absorption
ZELAPAR® disintegrates within seconds after placement on the tongue and is rapidly absorbed. Detectable levels of selegiline from ZELAPAR® have been measured at 5 minutes after administration, the earliest time point examined.
Selegiline is more rapidly absorbed from the 1.25 or 2.5 mg dose of ZELAPAR® (Tmax range: 10-15 minutes) than from the swallowed 5 mg selegiline tablet (Tmax range: 40-90 minutes). Mean (SD) maximum plasma concentrations of 3.34 (1.68) and 4.47 (2.56) ng/mL are reached after single dose of 1.25 and 2.5 mg ZELAPAR® compared to 1.12 ng/mL (1.48) for the swallowed 5 mg selegiline tablets (given as 5 mg bid). On a dose-normalized basis, the relative bioavailability of selegiline from ZELAPAR® is greater than from the swallowed formulation.
The pre-gastric absorption from ZELAPAR® and the avoidance of first-pass metabolism results in higher concentrations of selegiline and lower concentrations of the metabolites compared to the 5 mg swallowed selegiline tablet.
Plasma Cmax and AUC of ZELAPAR® were dose proportional at doses between 2.5 and 10 mg daily.
Food effects
When ZELAPAR® is taken with food, the Cmax and AUC of selegiline are about 60% of those seen when ZELAPAR® is taken in the fasted state. Since ZELAPAR® is placed on the tongue and absorbed through the oral mucosa (see DOSAGE AND ADMINISTRATION section), the intake of food and liquid should be avoided 5 minutes before and after ZELAPAR® administration.
Distribution
Up to 85% of plasma selegiline is reversibly bound to proteins.
Metabolism
Following a single dose, the median elimination half-life of selegiline was 1.3 hours at the 1.25 mg dose. Under steady-state conditions, the median elimination half-life increases to 10 hours. Upon repeat dosing, accumulation in the plasma concentration of selegiline is observed both with ZELAPAR® and the swallowed 5 mg tablet. Steady state is achieved after 8 days.
Selegiline is metabolized in vivo to 1-methamphetamine and desmethylselegiline and subsequently to 1-amphetamine; which in turn are further metabolized to their hydroxymetabolites.
ZELAPAR® also produces a smaller fraction of the administered dose recoverable as the metabolites than the conventional, swallowed formulation of selegiline.
In vitro metabolism studies indicate that CYP2B6 and CYP3A4 are involved in the metabolism of selegiline. CYP2A6 may play a minor role in the metabolism.
Elimination
Following metabolism in the liver, selegiline is excreted primarily in the urine as metabolites (mainly as L-methamphetamine) and as a small amount in the feces.
Special Populations
Age
The effect of age on the pharmacokinetics of selegiline following ZELAPAR® administration has not been adequately characterized.
Gender
There are no differences between male and female subjects in overall (AUC∞), time to maximum exposure (Tmax), and elimination half-life (t½) after administration of ZELAPAR®. Female subjects have an approximate 25% decrease in Cmax compared to male subjects. However, since the overall exposure (AUC∞) is not different between the genders, this pharmacokinetic difference is not likely to be clinically relevant.
Race
No studies have been conducted to evaluate the effects of race on the pharmacokinetics of ZELAPAR®.
Hepatic/Renal Impairment
No studies have been conducted to evaluate the pharmacokinetics of ZELAPAR® in hepatically- or renally-impaired patients. ZELAPAR® should be used with caution in patients with a history of or suspected renal or hepatic disease. (See PRECAUTIONS.)
Drug Interactions
No studies have been conducted to evaluate drug interactions on the pharmacokinetics of ZELAPAR®.
Effect of CYP3A inhibitor itraconazole
Itraconazole (200 mg QD) did not affect the pharmacokinetics of selegiline (single 10 mg oral, swallowed dose).
Although adequate studies have not been done investigating the effect of CYP3A4-inducers on selegiline, drugs that induce CYP3A4 (e.g. phenytoin, carbamazepine, nafcillin, phenobarbital, and rifampin) should be used with caution.
In vitro studies have demonstrated that selegiline is not an inhibitor of CYP450 enzymes. The induction potential of selegiline has not been adequately characterized. (See PRECAUTIONS, DRUG INTERACTIONS.)
CLINICAL STUDIES
The effectiveness of ZELAPAR® as an adjunct to levodopa/carbidopa in the treatment of Parkinson's disease was established in a multicenter randomized placebo-controlled trial (n=140; 94 received ZELAPAR®, 46 received placebo) of three months' duration. Patients randomized to ZELAPAR® received a daily dose of 1.25 mg for the first 6 weeks and a daily dose of 2.5 mg for the last 6 weeks. Patients were all treated with concomitant levodopa products and could additionally have been on concomitant dopamine agonists, anticholinergics, amantadine, or any combination of these during the trial. COMT (catechol-O-methyl-transferase) inhibitors were not allowed.
Patients with idiopathic Parkinson's disease receiving levodopa were enrolled if they demonstrated an average of at least 3 hours of "OFF" time per day on weekly diaries collected during a 2-week screening period. The patients enrolled had a mean duration of Parkinson's disease of 7 years, with a range from 0.3 years to 22 years.
At selected times during the 12 week study, patients were asked to record the amount of "OFF," "ON," "ON with dyskinesia," or "sleep" time per day for two separate days during the week prior to each scheduled visit. The primary efficacy outcome was the reduction in average percentage daily "OFF" time during waking hours from baseline to the end of the trial (averaging results at Weeks 10 and 12). Both treatment groups had an average of 7 hours per day of "OFF" time at baseline. The absolute mean percent reduction of "OFF" time was 13.1% for ZELAPAR® and 5.1% for placebo. ZELAPAR®-treated patients had an average of 2.2 hours per day less "OFF" time compared to baseline. Placebo-treated patients had 0.6 hours per day less "OFF" time compared to baseline. These differences were statistically significant (p < 0.001). Figure 1 shows the mean daily % "OFF" time during treatment over the whole study period for patients treated with ZELAPAR® vs. patients treated with placebo.
Figure 1
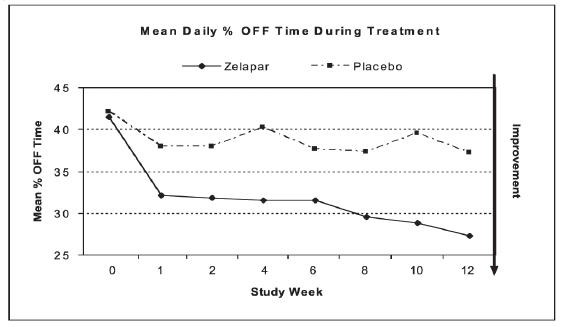
Dosage reduction of levodopa was allowed during this study if dopaminergic side effects, including dyskinesia and hallucinations, emerged. Levodopa dosage reduction occurred in 17% of patients in the ZELAPAR® group and in 19% in the placebo group. In those patients who had levodopa dosage reduced, the dose was reduced on average by 24% in the ZELAPAR® group and by 21% in the placebo group.
No difference in effectiveness based on age (patients > 66 years old vs. < 66 years) was detected. The treatment effect size in males was twice that in females, but, given the size of this single trial, this finding is of doubtful significance.
ZELAPAR INDICATIONS AND USAGE
ZELAPAR® is indicated as an adjunct in the management of patients with Parkinson's disease being treated with levodopa/carbidopa who exhibit deterioration in the quality of their response to this therapy. There is no evidence from controlled studies that ZELAPAR® has any beneficial effect in the absence of concurrent levodopa therapy.
ZELAPAR CONTRAINDICATIONS
ZELAPAR® is contraindicated in patients with a known hypersensitivity to any formulation of selegiline or any of the inactive ingredients of ZELAPAR®.
Meperidine and Other Analgesics
ZELAPAR® is contraindicated for use with meperidine. Serious reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors. These reactions have been characterized by coma, severe hypertension or hypotension, severe respiratory depression, convulsions, malignant hyperpyrexia, excitation, peripheral vascular collapse and death. At least 14 days should elapse between discontinuation of ZELAPAR® and initiation of treatment with meperidine. (See PRECAUTIONS-DRUG INTERACTIONS.)
For similar reasons, ZELAPAR® should not be administered with the analgesic agents tramadol, methadone, and propoxyphene.
Dextromethorphan
ZELAPAR® should not be used with the antitussive agent dextromethorphan. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior.
MAO inhibitors
ZELAPAR® should not be administered along with other selegiline products (e.g., ELDEPRYL and other tradenames) because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis. At least 14 days should elapse between discontinuation of ZELAPAR® and initiation of treatment with other selegiline products.
WARNINGS
ZELAPAR® should not be used at daily doses exceeding those recommended (2.5 mg/day) because of the risks associated with non-selective Inhibition of MAO. (See CLINICAL PHARMACOLOGY.)
The selectivity of ZELAPAR® for MAO-B may not be absolute even at the recommended daily dose of 2.5 mg a day. Even for "selective" MAO-B inhibitors, the selectivity for inhibiting MAO-B typically diminishes and is ultimately lost as the dose is increased beyond particular dose levels. Rare cases of hypertensive reactions associated with ingestion of tyramine containing foods have been reported even in patients taking the recommended daily dose of swallowed selegiline, a dose which is generally believed to be selective for MAO-B. Obviously, any selectivity is further diminished with increasing daily doses. An increase in tyramine sensitivity for blood pressure responses appears to occur beginning at a 5 mg daily dose. However, the precise dose at which ZELAPAR® becomes a non-selective inhibitor of all MAO is unknown.
Coadministration with Antidepressants
Severe CNS toxicity associated with hyperpyrexia and death has been reported with the combination of tricyclic antidepressants and non-selective MAOIs (NARDIL, PARNATE) or a selective MAO-B inhibitor, swallowed selegiline (ELDEPRYL). These adverse events have included behavioral and mental status changes, diaphoresis, muscular rigidity, hypertension, syncope, and death.
Serious, sometimes fatal, reactions with signs and symptoms including hyperthermia, rigidity, myoclonus, autonomic instability with rapid vital sign fluctuations, and mental status changes progressing to extreme agitation, delirium, and coma have been reported in patients receiving a combination of selective serotonin reuptake inhibitors (SSRIs), including fluoxetine (PROZAC), fluvoxamine (LUVOX), sertraline (ZOLOFT), and paroxetine (PAXIL) and non-selective MAOIs or the selective MAO-B inhibitor selegiline. Similar reactions have been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs) including venlafaxine and non-selective MAOIs or the selective MAO-B inhibitor selegiline.
Since the mechanisms of these reactions are not fully understood, it seems prudent, in general, to avoid these combinations of ZELAPAR® and tricyclic antidepressants as well as ZELAPAR® and serotonin reuptake inhibitors. Because of the long half-lives of fluoxetine and its active metabolite, at least five weeks (perhaps longer, especially if fluoxetine has been prescribed chronically and/or at higher doses) should elapse between discontinuation of fluoxetine and initiation of treatment with ZELAPAR®.
Orthostatic Hypotension
Although the incidence of orthostatic/postural hypotension reported as an adverse event was not higher in all patients treated in two clinical controlled trials, the incidence of adverse orthostatic hypotension was higher in geriatric patients (≥ 65 year old ) than in non-geriatric patients. In the geriatric patients, this adverse event of orthostatic hypotension occurred in about 3% of ZELAPAR®-treated patients compared to none (0%) of placebo-treated geriatric patients. Of potential relevance, the risk of dizziness was also greater in geriatric patients. In non-geriatric patients, the incidence of adverse orthostatic hypotension was not more frequent with ZELAPAR® than with placebo treatment.
Assessments of orthostatic (supine vs. standing) blood pressures at different times throughout the 12 week study period in two controlled trials showed that the frequency of orthostatic hypotension (> 20 mm Hg decrease in systolic blood pressure and/or > 10 mm Hg decrease in diastolic blood pressure) was greater with ZELAPAR® treatment than with placebo treatment. Of particular note, the treatment difference incidence (i.e. ZELAPAR® % - placebo %) of systolic and diastolic orthostatic decrements was most striking at 8 weeks (2 weeks after initiating 2.5 mg ZELAPAR®). At that time, the incidence of systolic orthostatic hypotension was about 21% in the ZELAPAR® patients and about 9% in the placebo patients. The incidence of diastolic orthostatic hypotension was about 12% in the ZELAPAR® group and about 4% in the placebo group. Thus, it appears that there may be an increased risk for orthostatic hypotension in the period after increasing the daily dose of ZELAPAR® from 1.25 to 2.5 mg.
PRECAUTIONS
Melanoma
Epidemiological studies have shown that patients with Parkinson's disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population.
Whether the increased risk observed was due to Parkinson's disease or other factors, such as drugs used to treat Parkinson's disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using ZELAPAR® for any indication. Ideally, periodic skin examination should be performed by appropriately qualified individuals (e.g., dermatologists).
General
Some patients given ZELAPAR® may experience an exacerbation of levodopa associated side effects, presumably due to the increased amounts of dopamine reacting with super sensitive, post-synaptic receptors. These effects may often be mitigated by reducing the dose of levodopa/carbidopa. For example, in the study demonstrating the efficacy of ZELAPAR®, there was an average 24% reduction in levodopa/carbidopa dosage in the 17% of patients who experienced a dose reduction during ZELAPAR® treatment.
The decision to prescribe ZELAPAR® should take into consideration that the MAO system of enzymes is complex and incompletely understood and there is only a limited amount of carefully documented clinical experience with ZELAPAR®. Consequently, the full spectrum of possible responses to ZELAPAR® may not have been observed in pre-marketing evaluation of the drug. It is advisable, therefore, to observe patients closely for atypical responses.
Phenylketonurics
It is important to note that each ZELAPAR® tablet contains 1.25 mg phenylalanine (a component of aspartame). Patients taking the 2.5 mg dose of ZELAPAR® will receive 2.5 mg phenylalanine.
Irritation of the Buccal Mucosa
In the controlled clinical trials, periodic examinations of the tongue and oral mucosa were performed. There was an increased frequency of mild oropharyngeal abnormality (e.g. swallowing pain, mouth pain, discrete areas of focal reddening, multiple foci of reddening, edema, and/or ulceration) at the end of the study in patients who did not have any abnormality at baseline and who received treatment with ZELAPAR® (10%) compared to patients who received placebo (3%). Separate analyses of each oropharyngeal abnormality were also assessed. ZELAPAR® patients (3%) showed an increased frequency of the development of mild discrete areas of focal reddening compared to placebo (0%) patients. ZELAPAR® patients (2%) also showed an increased frequency of the development of mild ulceration compared to placebo (1%) patients.
Dyskinesia
ZELAPAR® may potentiate the dopaminergic side effects of levodopa and may cause or exacerbate preexisting dyskinesia. Decreasing the dose of levodopa may ameliorate this side effect.
Effect on Renal Function
Small increments in serum BUN and creatinine have been observed in patients treated with ZELAPAR® 10 mg daily (4 times the recommended dose). Similar changes were not observed in patients treated with 1.25 or 2.5 mg daily.
Renally-Impaired Patients
The effect of ZELAPAR® has not been studied in renally-impaired patients. ZELAPAR® should therefore be used with caution in patients with a history of, suspected, or known renal impairment. If such patients experience adverse reactions that seem more frequent or severe than might ordinarily be expected, consideration should be given to discontinuing ZELAPAR®.
Hepatically-Impaired Patients
The effect of ZELAPAR® has not been studied in hepatically-impaired patients. ZELAPAR® should therefore be used with caution in patients with a history of, suspected, or known hepatic impairment, particularly if the patient has an increased prothrombin time or increased serum bilirubin or decreased serum albumin. If such patients experience adverse reactions that seem more frequent or severe than might ordinarily be expected, consideration should be given to discontinuing ZELAPAR®.
Withdrawal-Emergent Hyperpyrexia and Confusion
Although not reported with ZELAPAR® in the clinical development program, a symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
Hallucinations
When used as an adjunct to levodopa, hallucinations were reported as an adverse event in approximately 4% of patients treated with ZELAPAR® and 2% of patients treated with placebo. Hallucinations led to drug discontinuation and premature withdrawal from clinical trials in about 1% of patients treated with ZELAPAR® and none of the placebo treated patients.
Patients should be cautioned of the possibility of developing hallucinations and instructed to report them to their health care provider promptly should they develop.
Information for Patients
Patients should be advised of the possible need to reduce levodopa dosage after the initiation of ZELAPAR® therapy.
Patients (or their families if the patient is incompetent) should be advised not to exceed the daily recommended dose of 2.5 mg. The risk of using higher daily doses of ZELAPAR® should be explained, and a brief description of the hypertensive/cheese reaction provided. Rare hypertensive reactions with oral selegiline at recommended doses associated with dietary influences have been reported.
Consequently, it may be useful to inform patients (or their families) about the signs and symptoms associated with MAOI-induced hypertensive reactions. In particular, patients should be urged to report, immediately, any severe headache or other atypical or unusual symptoms not previously experienced.
Patients should be informed that hallucinations can occur.
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson's disease, including ZELAPAR®. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with ZELAPAR®. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges or other intense urges while taking ZELAPAR®. Physicians should consider dose reduction or stopping the medication if patient develops such urges while taking ZELAPAR®.
Patients should be instructed not to remove the blister from the outer pouch until just prior to dosing. The blister pack should then be peeled open with dry hands and the orally disintegrating tablet placed on the tongue, where it will disintegrate. Patients should also avoid drinking liquids or eating food five minutes before and after taking ZELAPAR®.
Laboratory Tests
No specific laboratory tests are deemed essential for the management of patients on ZELAPAR®. Periodic routine evaluation of all patients, however, is appropriate.
Drug Interactions
Meperidine
Serious, sometimes fatal reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors. (See CONTRAINDICATIONS )
Dextromethorphan
The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Therefore, in view of ZELAPAR®'s MAO inhibitory activity, dextromethorphan should not be used concomitantly with ZELAPAR®. (See CONTRAINDICATIONS.)
Selegiline Products
ZELAPAR® should not be administered along with other selegiline products (e.g., ELDEPRYL) because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis. (See CONTRAINDICATIONS.)
Sympathomimetic medications
One case of hypertensive crisis has been reported in a patient taking the recommended dose of swallowed selegiline and a sympathomimetic medication (ephedrine).
Tricyclic Antidepressants and Selective Serotonin Reuptake Inhibitors
Severe toxicity has also been reported in patients receiving the combination of tricyclic antidepressants and swallowed selegiline and selective serotonin reuptake inhibitors and swallowed selegiline. (See WARNINGS ).
Levodopa/carbidopa
(See PRECAUTIONS, General; PRECAUTIONS, Dyskinesia.)
Cytochrome P450 Enzymes
CYP2B6 and CYP3A4 are involved in the metabolism of selegiline. CYP2A6 may have a minor role in the metabolism of selegiline.
Effect of the CYP3A inhibitor itraconazole
Itraconazole (200 mg QD) did not affect the pharmacokinetics of selegiline (single 10 mg oral, swallowed dose).
Drugs that induce CYP450
Although adequate studies have not been done investigating the effect of CYP3A4-inducers on selegiline, drugs that induce CYP3A4 (e.g. phenytoin, carbamazepine, nafcillin, phenobarbital, and rifampin) should be used with caution.
Drug Interaction Studies
No drug interaction studies have been conducted to evaluate the effects of other drugs on the pharmacokinetics of ZELAPAR® or the effect of selegiline on other drugs. In vitro studies have demonstrated that selegiline is not an inhibitor of CYP450 enzymes. The induction potential of selegiline has not been adequately characterized. Drugs that induce CYP3A4 (phenytoin, carbamazepine, nafcillin, phenobarbital, and rifampin) should be used with caution.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Carcinogenicity studies of selegiline have not been conducted using the buccal route.
Selegiline did not induce mutations or chromosomal damage when tested in the bacterial mutation assay in Salmonella typhimurium and in an oral in vivo chromosomal aberration assay. While these studies provide some reassurance that selegiline is not mutagenic or clastogenic, they are not definitive because of methodological limitations. No definitive in vitro chromosomal aberration or in vitro mammalian gene mutation studies have been performed.
The effect of selegiline on fertility has not been adequately assessed.
Pregnancy
Teratogenic Effects
Pregnancy Category C
No teratogenic effects were observed in a study of embryo-fetal development in Sprague–Dawley rats at oral doses of 4, 12, and 36 mg/kg.
No teratogenic effects were observed in a study of embryo-fetal development in New Zealand White rabbits at oral doses of 5, 25, and 50 mg/kg; however, in this study, the number of litters produced at the two higher doses was less than recommended for assessing teratogenic potential.
In the rat study, there was a decrease in fetal body weight at the highest dose tested. In the rabbit study, increases in the total resorptions and percent post-implantation loss, and a decrease in the number of live fetuses per dam occurred at the highest dose tested.
In a peri- and post-natal development study in Sprague–Dawley rats (oral doses of 4, 16, and 64 mg/kg), an increase in the number of stillbirths and decreases in the number of pups per dam, pup survival, and pup body weight (at birth and throughout the lactation period) were observed at the two highest doses. At the highest dose tested, no pups born alive survived to Day 4 postpartum. Postnatal development at the highest dose tested in dams could not be evaluated because of the lack of surviving pups. The reproductive performance of the untreated offspring was not assessed.
No reproductive and developmental toxicology studies have been conducted using the buccal route.
There are no adequate and well-controlled studies in pregnant women. ZELAPAR® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether selegiline is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ZELAPAR®, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in patients under 16 years of age have not been established.
Geriatric Use
The majority of patients (128/194; 66%) who received ZELAPAR® in the double-blind placebo-controlled studies were 65 years of age and older (i.e. geriatric patients). There was no appreciable difference in treatment response of ZELAPAR® in geriatric vs. non-geriatric patients. However, the overall frequency of adverse events and of certain types of adverse events was increased in geriatric patients compared to non-geriatric patients. (See INCIDENCE IN CONTROLLED CLINICAL TRIALS UNDER ADVERSE REATIONS ).
ZELAPAR ADVERSE REACTIONS
A total of 578 patients received ZELAPAR® in clinical trials. Because the controlled trials performed during premarketing development both used a titration design (1.25 mg per day for 6 weeks, followed by 2.5 mg per day for 6 weeks), with a resultant confounding of time and dose, it was impossible to adequately evaluate the effects of dose on the incidence of adverse events.
The most commonly observed adverse events, which were greater than placebo, reported in the double-blind, placebo-controlled trials during ZELAPAR® treatment were dizziness, nausea, pain, headache, insomnia, rhinitis, dyskinesia, back pain, stomatitis, and dyspepsia.
Of the 194 patients treated with ZELAPAR® in the double-blind, placebo-controlled trials, 5.2% discontinued due to adverse events compared to 1.0% of the 98 patients who received placebo. Events causing discontinuation of treatment included dizziness, chest pain, accidental injury, and myasthenia.
INCIDENCE IN CONTROLLED CLINICAL TRIALS
Table 1 lists the adverse events reported in the placebo-controlled trials after at least one dose of ZELAPAR® (incidence ≥ 2%). The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these frequency estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients may differ.
|
Treatment- Emergent Adverse Events |
||
|---|---|---|
| Body System/Adverse Event |
ZELAPAR®
 1.25/2.5 mg N=194 % |
Placebo  N=98 % |
| Body as a Whole | ||
| Back Pain | 5 | 3 |
| Chest Pain | 2 | 0 |
| Pain | 8 | 7 |
| Cardiovascular System | ||
| Hypertension | 3 | 2 |
| Digestive System | ||
| Constipation | 4 | 0 |
| Diarrhea | 2 | 1 |
| Dysphagia | 2 | 1 |
| Dyspepsia | 5 | 3 |
| Flatulence | 2 | 1 |
| Nausea | 11 | 9 |
| Stomatitis | 5 | 4 |
| Tooth Disorder | 2 | 1 |
| Vomiting | 3 | 0 |
| Hemic and Lymphatic System | ||
| Ecchymosis | 2 | 0 |
| Metabolic and Nutritional Disorders | ||
| Hypokalemia | 2 | 0 |
| Musculoskeletal System | ||
| Leg Cramps | 3 | 1 |
| Myalgia | 3 | 0 |
| Nervous System | ||
| Ataxia | 3 | 1 |
| Depression | 2 | 1 |
| Dizziness | 11 | 8 |
| Dry Mouth | 4 | 2 |
| Dyskinesia | 6 | 3 |
| Hallucinations | 4 | 2 |
| Headache | 7 | 6 |
| Insomnia | 7 | 4 |
| Somnolence | 3 | 2 |
| Tremor | 3 | 1 |
| Respiratory System | ||
| Dyspnea | 3 | 0 |
| Pharyngitis | 4 | 2 |
| Rhinitis | 7 | 6 |
| Skin and Appendages | ||
| Rash | 4 | 1 |
| Skin Disorders |
6 | 2 |
Treatment emergent adverse events were reported at a higher frequency by patients ≥ 65 years of age compared to patients <65 years old. Analysis of adverse event incidence in each group was conducted to calculate and compare relative risk ZELAPAR® % / Placebo%) for each treatment. The relative risk was ≥ 2 fold higher for ZELAPAR® treatment in the geriatric patients compared to the non-geriatric patients for hypertension, orthostatic/postural hypotension (See WARNINGS-orthostatic hypotension ), dizziness, somnolence, ECG abnormality, nausea, dyspepsia, abnormal dreams, anxiety, cheilitis, diarrhea, hyperkalemia, pharyngitis, flu syndrome, and infection.
No consistent differences in the incidences of adverse events were observed between male and female patients.
There were insufficient data to assess the impact of race on the incidence of adverse events.
Other Adverse Events Observed During all Clinical Trials
ZELAPAR® has been administered to 578 patients for whom complete adverse event data was captured during all clinical trials, only some of which were placebo controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. Similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. All reported events are included below except those already listed elsewhere in labeling, those too general to be informative, and those not reasonably associated with the use of the drug.
Body as a Whole: allergic reaction, cellulitis, cyst, face edema, fever, hernia, infection fungal, infection superimposed, infection viral, neck pain, neoplasm, pain flank, cyanosis.
Nervous System: abnormal gait, agitation, akinesia, aphasia, CNS neoplasia, dementia, dystonia, emotional lability, encephalopathy, hyperkinesias, hypertonia, hypokinesia, hypotonia, incoordination, increased salivation, myclonus, nervousness, neuralgia, neuropathy, paranoid reaction, paresthesia, peripheral neuritis, personality disorder, psychosis, reflexes decreased, sleep disorder, subdural hematoma, thinking abnormal, vertigo, migraine.
Digestive System: anorexia, cholecystitis, cholelithiasis, colitis, esophageal ulcer, esophagitis, gamma glutamyl transpeptidase increased, gastritis, gastroenteritis, gingivitis, hepatitis, intestinal obstruction, liver function test abnormal, peptic ulcer, tongue edema.
Cardiovascular System: angina pectoris, atrial fibrillation, atrial flutter, AV block first degree, bigeminy, cardiomegaly, cardiomyopathy, cerebral ischemia, congestive heart failure, heart arrest, hypotension, migraine, myocardial infarct, myocardial ischemia, pallor, sinus bradycardia, supraventricular tachycardia, syncope, vascular disorder, vasodilation.
Musculoskeletal System: arthralgia, arthritis, arthrosis, bone pain, bursitis, leg cramps, tendon rupture, tenosynovitis.
Respiratory System: sinusitis, asthma, bronchitis, carcinoma of the lung, hiccup, epistaxis, lung edema, pleural effusion, pneumonia, pneumothorax, voice alteration.
Skin and Appendages: contact dermatitis, dry skin, eczema, fungal dermatitis, herpes simplex, herpes zoster, pruritis, seborrhea, skin benign neoplasm, skin carcinoma, skin hypertrophy, skin melanoma, skin discoloration, skin ulcer, sweating.
Metabolic and Nutritional Disorders: avitaminosis, dehydration, diabetes mellitus, edema, gout, hyperchloestermia, hyperglycemia, hyperkalemia, hyperlipidemia, hyperphosphatemia, hypoglycemia, albuminuria, hyponatremia, hypoproteinemia, SPGT increased.
Urogenital Disorders: breast carcinoma, cystitis, epididymitis, kidney calculus, ovarian disorder, prostatic carcinoma, prostatic specific antigen increase, urinary frequency, urination impaired, urinary incontinence, urinary urgency.
Special Senses: abnormal vision, amblyopia, blindness, cataract specified, conjunctivitis, deafness, diplopia, dry eyes, eye hemorrhage, glaucoma, otitis externa, retinal artery occlusion, retinal detachment, taste loss, taste perversion, tinnitus.
Hemic and Lymphatic System: abnormal platelets, anemia, chronic leukocytosis, cyanosis, eosinophilia, lymphoma like reaction, myelocytic leukemia, sedimentation rate increased.
Postmarketing Reports
There have been reports of the following adverse reactions: pathological gambling, increased libido including hypersexuality, and impulse control symptoms while taking one or more of the medications that are generally used for the treatment of Parkinson's disease.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a casual relationship to drug exposure.
OVERDOSAGE
Selegiline
No specific information is available about clinically significant overdoses with swallowed selegiline or ZELAPAR®. However, experience gained during development of the 5 mg swallowed dosage form reveals that some individuals exposed to doses of 600 mg of d,l-selegiline suffered severe hypotension and psychomotor agitation.
Since the selective inhibition of MAO-B by ZELAPAR® is achieved only at doses in the range recommended for the treatment of Parkinson's disease (e.g., 2.5 mg/day), overdoses are likely to cause significant inhibition of both MAO-A and MAO-B. Consequently, the signs and symptoms of overdose may resemble those observed with marketed non-selective MAO inhibitors [e.g., tranylcypromine (PARNATE), isocarboxazide (MARPLAN), and phenelzine (NARDIL)]. For this reason, in cases of overdose with selegiline, dietary tyramine restriction should be observed for several weeks to avoid the risk of a hypertensive/cheese reaction.
Overdose with Non-Selective MAO Inhibitors
NOTE: This section is provided for reference; it does not describe events that have actually been observed with oral selegiline or ZELAPAR® in overdose.
Characteristically, signs and symptoms of non-selective MAOI overdose may not appear immediately. Delays of up to 12 hours between ingestion of drug and the appearance of signs may occur. Importantly, the peak intensity of the syndrome may not be reached for upwards of a day following the overdose. Death has been reported following overdosage. Therefore, immediate hospitalization, with continuous patient observation and monitoring for a period of at least two days following the ingestion of such drugs in overdose, is strongly recommended.
The clinical picture of MAOI overdose varies considerably; its severity may be a function of the amount of drug consumed. The central nervous and cardiovascular systems are prominently involved.
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
Treatment Suggestions for Overdose
NOTE: Because there is no recorded experience with swallowed selegiline or ZELAPAR® overdose, the following suggestions are offered based upon the assumption that such overdoses may be modeled by non-selective MAOI poisoning. In any case, up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Poison Control Centers are listed in the Physicians Desk Reference (PDR).
Treatment of overdose with non-selective MAOIs is symptomatic and supportive. Induction of emesis or gastric lavage with instillation of charcoal slurry may be helpful in early poisoning, provided the airway has been protected against aspiration. Signs and symptoms of central nervous system stimulation, including convulsions, should be treated with diazepam, given slowly intravenously. Phenothiazine derivatives and central nervous system stimulants should be avoided. Hypotension and vascular collapse should be treated with intravenous fluids and, if necessary, blood pressure titration with an intravenous infusion of a dilute pressor agent. It should be noted that adrenergic agents may produce a markedly increased pressor response.
Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required.
Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential.
ZELAPAR DOSAGE AND ADMINISTRATION
ZELAPAR® is intended for administration to patients with Parkinson's disease receiving levodopa/carbidopa therapy who demonstrate a deteriorating response to this treatment.
Treatment should be initiated with 1.25 mg given once a day for at least 6 weeks. After 6 weeks, the dose may be escalated to 2.5 mg given once a day if a desired benefit has not been achieved and the patient is tolerating ZELAPAR®. There is no evidence that doses greater than 2.5 mg a day confer any additional benefit, and they should ordinarily be avoided because of the potential increased risk of adverse events.
ZELAPAR® should be taken in the morning before breakfast and without liquid.
In the controlled trial of ZELAPAR® in which ZELAPAR® was shown to be effective compared to placebo, 17% of patients in the ZELAPAR® group and 19% of patients in the placebo treatment group had a reduction in their doses of levodopa/carbidopa because of perceived dopaminergic side effects. For those patients with a dose reduction, the average reduction was 24% for ZELAPAR® and 21% for placebo.
Patients should not attempt to push ZELAPAR® through the foil backing. Patients should PEEL BACK the backing of one or two blisters (as prescribed) with dry hands, and GENTLY remove the tablet(s). Patients should IMMEDIATELY place the ZELAPAR® tablet(s) on top of the tongue where it will disintegrate in seconds. Patients should avoid ingesting food or liquids for 5 minutes before and after taking ZELAPAR®.
HOW SUPPLIED
ZELAPAR® Orally Disintegrating Tablets are available containing 1.25 mg selegiline hydrochloride in a Zydis® formulation. Each pale yellow tablet is imprinted with a stylized "V". Ten tablets are contained in a moisture-resistant pouch and packaged in a carton. Neither the blister card nor the pouch is child-resistant.
ZELAPAR® (selegiline hydrochloride) is available as: NDC 0187-0453-02, carton of 6 pouches (60 tablets).
STORAGE
Store at controlled room temperature, 25°C (77°F); excursions permitted to 15-30°C (59-86°F). Use within 3 months of opening pouch and immediately upon opening individual blister. Store blister tablets in pouch. Potency cannot be guaranteed after 3 months of opening the pouch.
Rx Only
ZELAPAR® (selegiline hydrochloride) 1.25 mg Orally Disintegrating Tablets are manufactured for:
Valeant Pharmaceuticals North America, Aliso Viejo, CA 92656, USA
By:
Catalent
Swindon, Wiltshire, SN5 8RU, UK
Issued Feb 2009
Printed in the UK
ZELAPAR is a registered trademark of Valeant Pharmaceuticals North America or its related companies. Zydis® is a registered trademark of Catalent Pharma Solutions or one of its subsidiaries, used under license. All other trademarks are the trademarks or registered trademarks of their respective owners.
Part No. 11EP3454D
Rev. February 2009
PRINCIPAL DISPLAY PANEL - 6 pouches, 10 Tablet Carton
NDC 0187-0453-02
Rx Only
Zelapar® 1.25 mg
(selegiline HCl)
Orally Disintegrating Tablets
6 pouches,
each containing
10 tablets
VALEANT®

Zelaparselegiline hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||