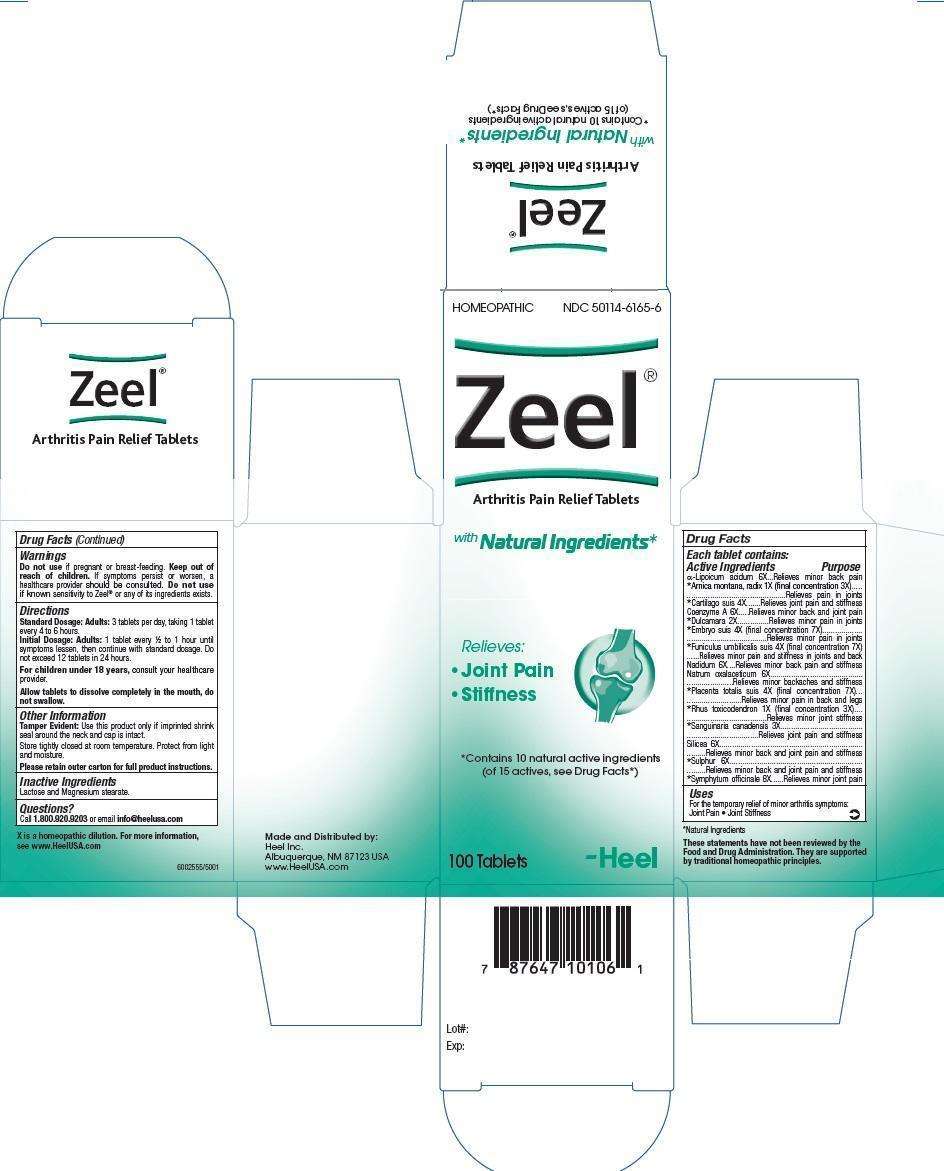
Zeel
Zeel Tablet
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
- ZEEL DOSAGE AND ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- ZEEL INDICATIONS AND USAGE
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Active Ingredients: Each tablet contains: α-Lipoicum acidum 6X, Arnica montana, radix 1X (final concentration 3X), Cartilago suis 4X, Coenzyme A 6X, Dulcamara 2X, Embryo suis 4X (final concentration 7X), Funiculus umbilicalis suis 4X (final concentration 7X), Nadidum 6X, Natrum oxalaceticum 6X, Placenta totalis suis 4X (final concentration 7X), Rhus toxicodendron 1X (final concentration 3X), Sanguinaria canadensis 3X, Silicea 6X, Sulphur 6X, Symphytum officinale 8X.
INACTIVE INGREDIENTS
Inactive Ingredients: Lactose and Magnessium stearate
PURPOSE
α-Lipoicum acidum 6X............................................Relieves minor back pain
Arnica montana, radix 1X (final concentration 3X)......Relieves pain in joints
Cartilago suis 4X....................................................Relieves joint pain and stiffness
Coenzyme A 6X.....................................................Relieves minor back and joint pain
Dulcamara 2X.......................................................Relieves minor pain in joints
Embryo suis 4X (final concentration 7X)....................Relieves minor pain in joints
Funiculus umbilicalis suis 4X (final concentration 7X)..Relieves minor pain and stiffness in joints and back
Nadidum 6X..........................................................Relieves minor back pain and stiffness
Natrum oxalaceticum 6X.........................................Relieves minor backaches and stiffness
Placenta totalis suis 4X (final concentration 7X).........Relieves minor pain in back and legs
Rhus toxicodendron 1X (final concentration 3X).........Relieves minor joint stiffness
Sanguinaria canadensis 3X.....................................Relieves joint pain and stiffness
Silicea 6X.............................................................Relieves minor back and joint pain and stiffness
Sulphur 6X...........................................................Relieves minor back and joint pain and stiffness
Symphytum officinale 8X........................................Relieves minor joint pain
ZEEL DOSAGE AND ADMINISTRATION
Standard Dosage: Adults: 3 tablets per day, taking 1 tablet every 4 to 6 hours.
Initial Dosage: Adults: 1 tablet every 1/2 to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceed 12 tablets in 24 hours.
For children under 18 years, consult your healthcare provider.
Allow tablets to dissolve completely in the mouth, do not swallow.
WARNINGS
Do not use if pregnant or breast-feeding. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to Zeel® or any of its ingredients exists.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
ZEEL INDICATIONS AND USAGE
For the temporary relief of minor arthritis symptoms:
- Joint Pain
- Joint Stiffness
Zeel.ALPHA.-LIPOIC ACID, ARNICA MONTANA ROOT, SUS SCROFA CARTILAGE, COENZYME A, SOLANUM DULCAMARA TOP, SUS SCROFA EMBRYO, SUS SCROFA UMBILICAL CORD, NADIDE, SODIUM DIETHYL OXALACETATE, SUS SCROFA PLACENTA, TOXICODENDRON PUBESCENS LEAF, SANGUINARIA CANADENSIS ROOT, SILICON DIOXIDE, SULFUR and COMFREY ROOT TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||