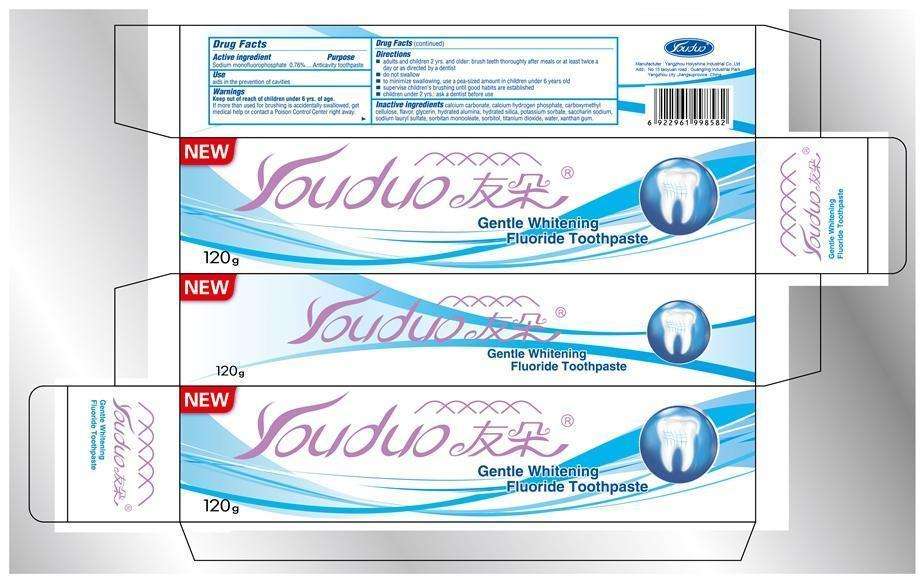
Youduo Gentle Whitening Fluoride
Yangzhou Holyshine Industrial Co., Ltd.
Youduo Gentle Whitening Fluoride Toothpaste
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Sodium monofluorophosphate 0.76%
Purpose
Anticavity toothpaste
Use
aids in the prevention of cavities
Warnings
Keep out of reach of children under 6 yrs of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 yrs. and older: brush teeth thoroughly after meals or at least twice a day or as directed by a dentist.
- do not swallow
- to minimize swallowing, use a pea-sized amount in children under 6 years old
- supervise children's brushing until good habits are established
- children under 2yrs.: ask a dentist before use.
Inactive ingredients
calcium carbonate, calcium hydrogen phosphate, carboxymethyl cellulose, flavor, glycerin, hydrated alumina, hydrated silica, potassium sorbate, saccharin sodium, sodium lauryl sulfate, sorbitan monooleate, sorbitol, titanium dioxide, water, xanthan gum.
Manufacturer : Yangzhou Holyshine Industrial Co., Ltd.
Add: No 15 taoyuan raod , Guangling Industrail Park,
Yangzhou city .Jiangsuprovice ,China
NEW Youduo
Gentle Whitening Fluoride Toothpaste
120g
Youduo Gentle Whitening FluorideSODIUM MONOFLUOROPHOSPHATE PASTE, DENTIFRICE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||