Wart Remover Liquid
Premier Brands of America Inc.
Wart Remover Liquid
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Wart Remover Liquid
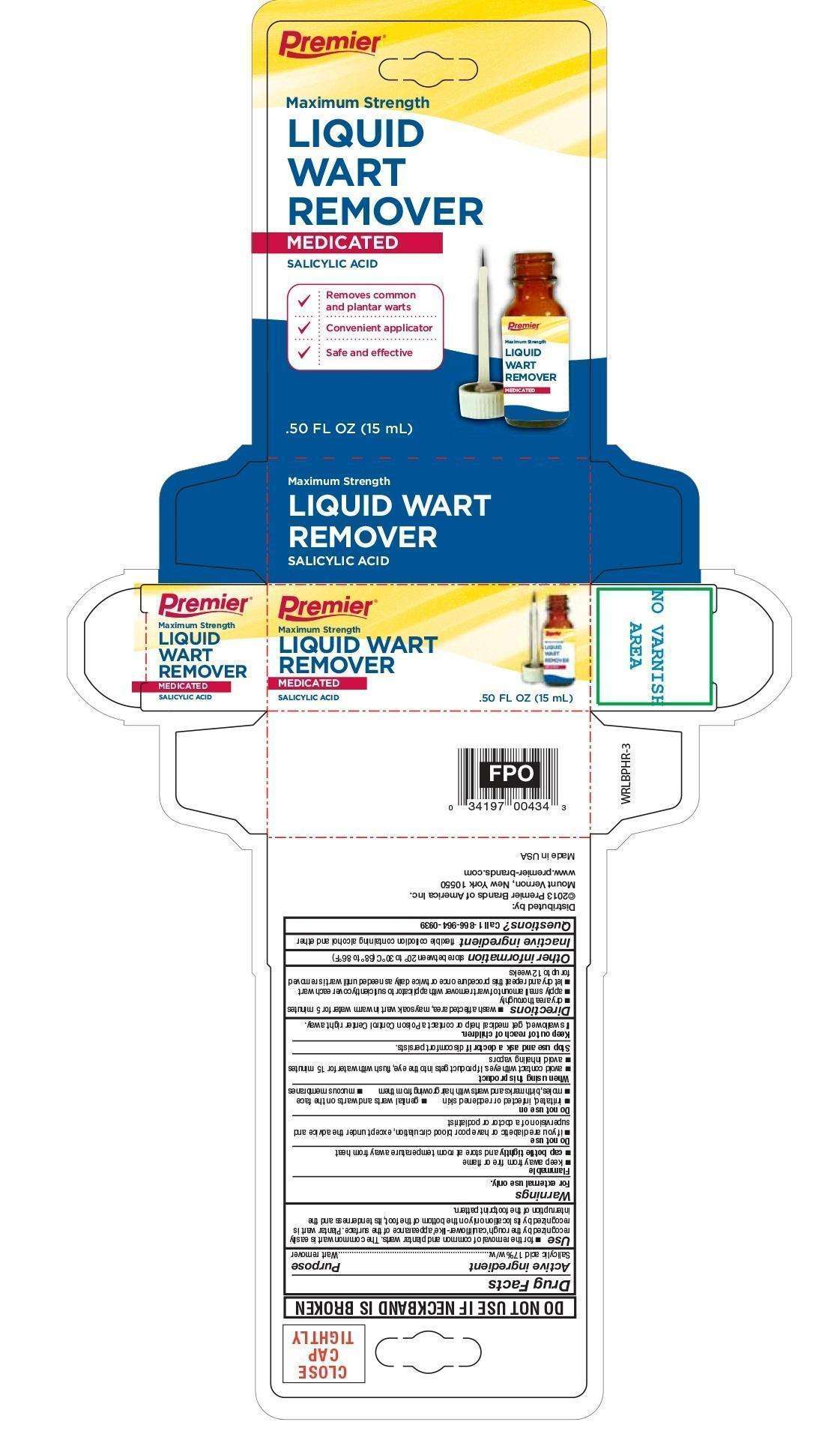

Salicylic acid 17%w/w
Wart remover
- for the removal of common and plantar warts. The common wart is easily recognized by the rough 'cauliflower-like' appearance of the surface. Plantar wart is recognized by its location only on the bottom of the foot, its tenderness and the interruption of the footprint pattern.
For external use only.
- keep away from fire or flame
- cap bottle tightly and store at room temperature away heat
- if you are a diabetic or have poor blood circulation, except under the advice and supervision of a doctor or podiatrist
- irritated, infected or reddened skin
- genital warts and warts on the face
- moles, birthmarks and warts with hair growing from them
- mucous membranes
- avoid contact with eyes. If product gets into the eyes, flush with water for 15 minutes
- avoid inhaling vapors
discomfort persists
If swallowed, get medical help or contact a Poison Control Center right away.
- wash affected area, may soak wart in warm water for 5 minutes
- dry area thoroughly
- apply small amount of wart remover with applicator to sufficiently cover each wart
- let dry and repeat this procedure once or twice daily until wart is removed for up to 12 weeks
store between 20°C to 30°C (68°F to 86°F)
camphor, castro oil, ethanol, ethyl ether, nitrocellulose
Call 1-866-964-0939
Wart Remover LiquidWart Remover Liquid LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!