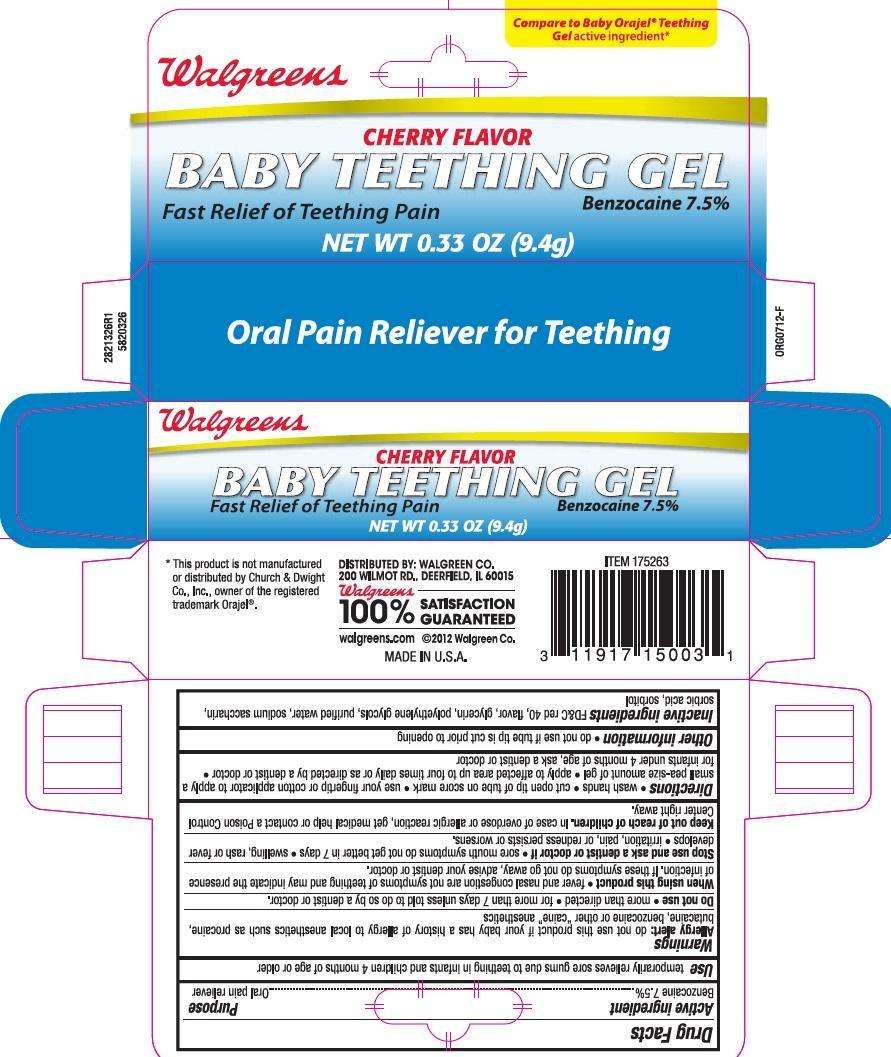
Walgreens
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Benzocaine 7.5% ...................................................................... Oral pain reliever
Use
temporarily relieves sore gums due to teething in infants and children 4 months of age and older
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as
procaine, butacaine, benzocaine or other “caine” anesthetics
- Do not use more than directed for more than 7 days unless told to do so by a dentist or doctor.
- When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms do not go away, advise your dentist or doctor.
- Stop use and ask a doctor if sore mouth symptoms do not get better in 7 days. swelling, rash or fever develops, irritation, pain or redness persists or worsens
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- wash hands
- cut open tip of tube on scoremark
- use your fingertip or cotton applicator to apply a small pea-size amount of gel
- apply to affected area up to four times daily or as directed by a dentist or doctor
- for infants under 4 months of age, ask a dentist or doctor
Other Information
Do not use if tip is cut prior to opening.
Inactive ingredients
FD&C Red 40, Flavor, Glycerin, Polyethylene Glycols, Purified Water, Sodium Saccharin, Sorbic Acid, Sorbitol
mm1.jpg
mm2.jpg

WalgreensBenzocaine GEL, DENTIFRICE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!