Walgreens Medicated Callus Removers
Walgreens Medicated Gel Callus Removers
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Walgreens Medicated Callus Removers Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
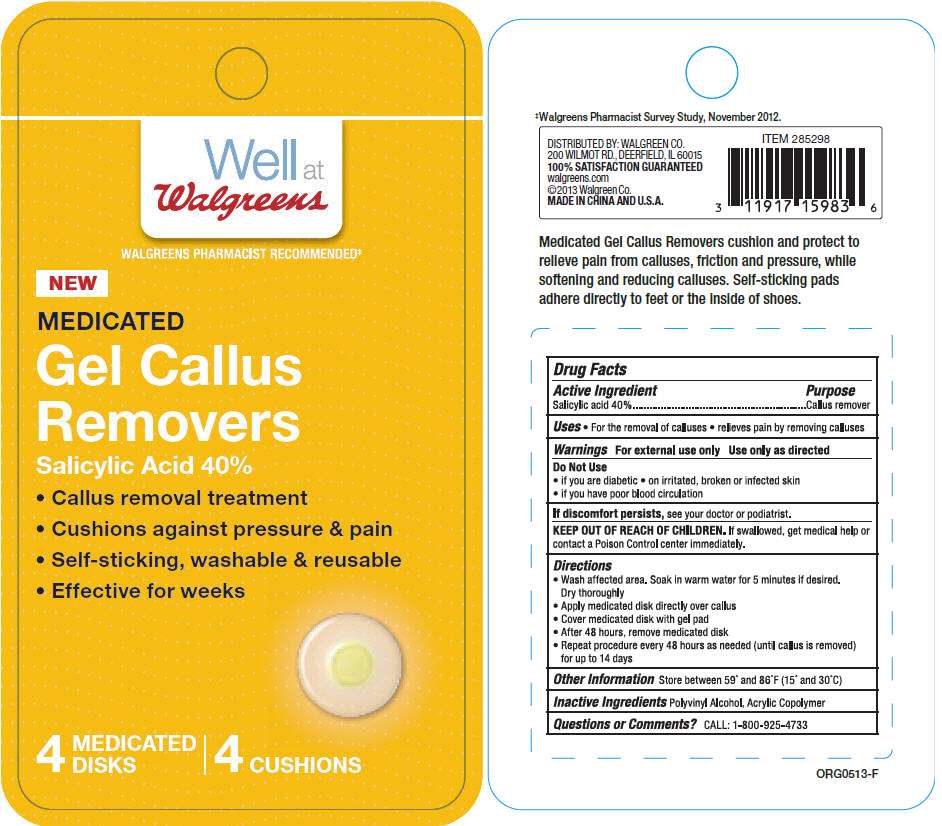
- PRINCIPAL DISPLAY PANEL - 4 Disk Blister Pack
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Salicylic acid 40%
Purpose
Callus remover
Walgreens Medicated Callus Removers Uses
- For the removal of calluses
- relieves pain by removing calluses
Warnings
For external use only Use only as directed
Do Not Use
- if you are diabetic
- on irritated, broken or infected skin
- if you have poor blood circulation
If discomfort persists, see your doctor or podiatrist.
KEEP OUT OF REACH OF CHILDREN. If swallowed, get medical help or contact a Poison Control center immediately.
Directions
- Wash affected area. Soak in warm water for 5 minutes if desired.
Dry thoroughly - Apply medicated disk directly over callus
- Cover medicated disk with gel pad
- After 48 hours, remove medicated disk
- Repeat procedure every 48 hours as needed (until callus is removed) for up to 14 days
Other Information
Store between 59° and 86°F (15° and 30°C)
Inactive Ingredients
Polyvinyl Alcohol, Acrylic Copolymer
Questions or Comments?
CALL: 1-800-925-4733
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
PRINCIPAL DISPLAY PANEL - 4 Disk Blister Pack
Well at
Walgreens
WALGREENS PHARMACIST RECOMMENDED‡
NEW
MEDICATED
Gel Callus
Removers
Salicylic Acid 40%
- Callus removal treatment
- Cushions against pressure & pain
- Self-sticking, washable & reusable
- Effective for weeks
4
MEDICATED
DISKS | 4 CUSHIONS
Walgreens Medicated Callus RemoversSALICYLIC ACID DISC
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||