Vivotif-B
Vivotif-B
FULL PRESCRIBING INFORMATION: CONTENTS*
- VIVOTIF-B DESCRIPTION
- CLINICAL PHARMACOLOGY
- VIVOTIF-B INDICATIONS AND USAGE
- VIVOTIF-B CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- VIVOTIF-B ADVERSE REACTIONS
- VIVOTIF-B DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
VIVOTIF-B DESCRIPTION
Vivotif (Typhoid Vaccine Live Oral Ty21a) is a live attenuated vaccine for oral administration only. The vaccine contains the attenuated strain Salmonella typhi Ty21a (1,2).
Vivotif is manufactured by the Berna Biotech Ltd. The vaccine strain is grown in fermentors under controlled conditions in medium containing a digest of yeast extract, an acid digest of casein, dextrose and galactose. The bacteria are collected by centrifugation, mixed with a stabilizer containing sucrose, ascorbic acid and amino acids, and lyophilized. The lyophilized bacteria are mixed with lactose and magnesium stearate and filled into gelatin capsules which are coated with an organic solution to render them resistant to dissolution in stomach acid. The
enteric-coated, salmon/white capsules are then packaged in 4-capsule blisters for distribution. The contents of each enteric-coated capsule are shown in Table 1.
Table 1: Contents of one enteric-coated capsule of Vivotif (Typhoid Vaccine Live Oral Ty21a)
Viable S. typhi Ty21a . . . . . . . . . . . . . . . . . . . . . . . . . 2–6.8x109 colony-forming units*
Non-viable S. typhi Ty21a . . . . . . . . . . . . . . . . . . . . . 5–50x109 bacterial cells
Sucrose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26–130 mg
Ascorbic acid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1–5 mg
Amino acid mixture . . . . . . . . . . . . . . . . . . . . . . . . . . 1.4–7 mg
Lactose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100–180 mg
Magnesium stearate . . . . . . . . . . . . . . . . . . . . . . . . . 3.6–4.4 mg
*Vaccine potency (viable cell counts per capsule) is determined by inoculation of agar plates with appropriate dilutions of the vaccine suspended in physiological saline.
CLINICAL PHARMACOLOGY
Salmonella typhi is the etiological agent of typhoid fever, an acute, febrile enteric disease. Typhoid fever continues to be an important disease in many parts of the world. Travelers entering infected areas are at risk of contracting typhoid fever following the ingestion of contaminated food or water. Typhoid fever is considered to be endemic in most areas of Central and South America, the African continent, the Near East and the Middle East, Southeast Asia and the Indian subcontinent (3). There are approximately 500 cases of typhoid fever per year diagnosed
in the United States (4). In 62 % of these patients (data from 1975–1984) the disease was acquired outside of the United States while in 38 % of the patients the disease was acquired within the United States (5). Of 340 cases acquired in the United States between 1977 and 1979, 23% of the cases were associated with typhoid carriers, 24% were due to food outbreaks, 23% were associated with the ingestion of contaminated food or water, 6% due to household contact with an infected person and 4% following exposure to S. typhi in a laboratory setting (6).
The majority of typhoid cases respond favorably to antibiotic therapy. However, the emergence of multi-drug resistant strains has greatly complicated therapy and cases of typhoid fever that are treated with ineffective drugs can be fatal (7). Approximately 2–4% of acute typhoid cases result in the development of a chronic carrier state (8). These non-symptomatic carriers are the natural reservoir for S. typhi and can serve to maintain the disease in its endemic state or to directly infect individuals (3).
Virulent strains of S. typhi upon ingestion are able to pass through the stomach acid barrier, colonize the intestinal tract, penetrate the lumen and enter the lymphatic system and blood stream, thereby causing disease. One possible mechanism by which disease may be prevented is by evoking a local immune response in the intestinal tract.
Such local immunity may be induced by oral ingestion of a live attenuated strain of S. typhi undergoing an aborted infection. The ability of S. typhi to cause disease and to induce a protective immune response is dependent upon the bacteria possessing a complete lipopolysaccharide (1). The S. typhi Ty21a vaccine strain, by virtue of a reduction
in enzymes essential for lipopolysaccharide biosynthesis, is restricted in its ability to produce complete lipopolysaccharide (1,2). However, a sufficient quantity of complete lipopolysaccharide is synthesized to evoke a protective immune response. Despite low levels of lipopolysaccharide synthesis, the cells lyse before regaining a virulent phenotype due to the intracellular build-up of intermediates during lipopolysaccharide synthesis (1,2).
Results from clinical studies indicate that adults and children greater than 6 years of age may be protected against typhoid fever following the oral ingestion of 4 doses of Vivotif (Typhoid Vaccine Live Oral Ty21a). The efficacy of the S. typhi Ty21a strain has been evaluated in a series of randomized, double-blind, controlled field trials. Suspected typhoid cases, detected by passive surveillance, were confirmed bacteriologically either by blood or bone marrow culture. The first trial was performed in Alexandria, Egypt with a study population of 32,388 children aged 6 to 7 years. Three doses of vaccine, in the form of a freshly reconstituted suspension administered after ingestion of 1 g of bicarbonate, were given on alternate days. Immunization resulted in a 95% decrease [95% confidence interval (CI) = 77%–99%] in the incidence of typhoid fever over a 3-year period of surveillance (9). A series of field trials were subsequently performed in Santiago, Chile to evaluate efficacy when the vaccine strain was administered in the form of an acid-resistant enteric-coated capsule. The initial trial involved 82,543 school-aged children, and compared 1 or 2 doses of vaccine given one week apart. After 24 months of surveillance vaccine efficacy was 29% (95% CI = 4%–47%) for the single dose schedule and 59% (95% CI = 41%–71%) for the 2-dose schedule (10). A further field trial was performed in Santiago, Chile involving 109,594 school-aged children (11). Three doses of enteric-coated capsules were administered either on alternate days (short immunization schedule) or 21 days apart (long immunization schedule). Following 36 months of surveillance vaccination resulted in a 67% (95% CI = 47%–79%) decrease in the incidence of typhoid fever in the short immunization schedule group and a 49% reduction (95% CI = 24%–66%) in the long immunization schedule group. After 48 months of surveillance the short immunization schedule resulted in a 69% (95% CI = 55%–80%) decrease in typhoid fever (12). An undiminished level of protection was observed during the fifth year of surveillance. A field trial was next conducted in Santiago,
Chile to determine the relative efficacy of 2, 3 and 4 doses of enteric-coated vaccine administered on alternate days to school-aged children. Relative vaccine efficacy as determined by comparison of disease incidence within the three vaccinated groups was highest for the four dose regimen (13). The incidence of typhoid fever per 105 study subjects was 160.5 (95% CI = 130–191) for the three dose regimen versus 95.8 (95% CI = 71–121) for the four dose regimen (p<0.004). An additional field trial to determine vaccine efficacy was conducted in Plaju, Indonesia involving
20,543 individuals approximately 3 to 44 years of age (14). Due to logistical considerations three doses of enteric-coated capsules were administered at weekly intervals, a schedule known to provide suboptimal protection (11). After 30 months of surveillance vaccine efficacy for all age groups was 42% (95% CI = 23%–57%).
Vaccine organisms can be shed transiently in the stool of vaccine recipients (16). However, secondary transmission of vaccine organisms has not been documented. Ty21a has not been isolated from blood cultures following immunization. At present, the precise mechanism(s) by which Vivotif confers protection against typhoid fever is unknown. However, it is known that immunization of adult subjects can elicit a humoral anti-S. typhi LPS antibody response. Taking advantage of this fact, the seroconversion rate (defined as a ≥0.15 increase in optical density
units over baseline determined in an ELISA) was compared in an open study between adults living in an endemic area (Chile) and non-endemic areas (United States and Switzerland) after the ingestion of 3 doses of vaccine.
Comparable seroconversion rates were seen between these groups (15). S. typhi Ty21a cultured in medium not containing BHI induced an anti-S. typhi LPS antibody response comparable to that obtained with vaccine organisms cultured in medium containing BHI (15). Challenge studies in North American volunteers have shown that the Ty21a strain is capable of providing significant protection to an experimental challenge of S. typhi (16).
Because of the very low incidence of typhoid fever in United States citizens, efficacy studies are not currently feasible in this population. However, the above observations support the expectation that Vivotif will provide protection to recipients from non-typhoid endemic areas such as the United States.
VIVOTIF-B INDICATIONS AND USAGE
Vivotif (Typhoid Vaccine Live Oral Ty21a) is indicated for immunization of adults and children greater than 6 years of age against disease caused by Salmonella typhi. Routine typhoid vaccination is not recommended in the United States of America. Selective immunization against typhoid fever is recommended for the following groups: 1) travelers to areas in which there is a recognized risk of exposure to S. typhi, 2) persons with intimate exposure (e.g. household contact) to a S. typhi carrier, and 3) microbiology laboratorians who work frequently with S. typhi
(7). There is no evidence to support the use of typhoid vaccine to control common source outbreaks, disease following natural disasters or in persons attending rural summer camps.
Not all recipients of Vivotif will be fully protected against typhoid fever. Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms. The vaccine will not afford protection against species of Salmonella other than Salmonella typhi or other bacteria that cause enteric disease. The vaccine is not suitable for treatment of acute infections with S. typhi.
VIVOTIF-B CONTRAINDICATIONS
Hypersensitivity to any component of the vaccine or the enteric-coated capsule. The vaccine should not be administered to persons during an acute febrile illness. Safety of the vaccine has not been demonstrated in persons deficient in their ability to mount a humoral or cell-mediated immune response, due to either a congenital or acquired immunodeficient state including treatment with immunosuppressive or antimitotic drugs. The vaccine should not be administered to these persons regardless of benefits.
WARNINGS
Vivotif (Typhoid Vaccine Live Oral Ty21a) is not to be taken during an acute gastrointestinal illness. The vaccine should not be administered to individuals receiving sulfonamides and antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response. Postpone taking the vaccine if persistent diarrhea or vomiting is occurring. Unless a complete immunization schedule is followed, an optimum immune response may not be achieved. Not all recipients of Vivotif will be fully protected against typhoid fever. Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms, i.e. travelers should take all necessary precautions to avoid contact or ingestion of potentially contaminated food or water.
Drug-Interactions
Several anti-malaria drugs, such as mefloquine, chloroquine and proguanil (not approved for use in US) possess anti-bacterial activity which may interfere with the immunogenicity of Vivotif (17,18). To determine the effect of these anti-malaria drugs on the humoral IgG or IgA anti-S. typhi immune response, healthy adult subjects were given mefloquine (250 mg at weekly intervals; N = 30) chloroquine (500 mg at weekly intervals; N = 30) or proguanil
(200 mg daily; N = 30) together with the S. typhi Ty21a vaccine strain (19). Concomitant treatment with mefloquine or chloroquine did not result in a significant reduction in the serum anti-S. typhi immune response compared to subjects receiving vaccine strain only (N = 45). The simultaneous administration of proguanil did effect a significant decrease in the immune response rate. These findings indicate that mefloquine and chloroquine can be administered together with Vivotif. Proguanil should be administered only if 10 days or more have elapsed since the final dose of Vivotif was ingested. The concomitant administration of oral polio vaccine or yellow fever vaccine does not suppress the immune response elicited by the Ty21a vaccine strain (19). There are no data regarding simultaneous administration of other parenteral vaccines or immunoglobulins with Vivotif.
PRECAUTIONS
General
The health care provider should take all necessary precautions to ensure the safe and effective use of the vaccine.
Patients should be questioned about previous reactions to this or similar products. The previous immunization history of the patient and current antibiotic usage should be obtained by the health care provider.
Information for Patients
It is essential that all 4 doses of vaccine be taken at the prescribed alternate day interval to obtain a maximal protective immune response. Vaccine potency is dependent upon storage under refrigeration [between 2 °C and 8 °C (35.6 °F– 46.4 °F)]. The vaccine should be stored under refrigeration at all times. It is essential to replace unused vaccine in the refrigerator between doses. The vaccine capsule should be swallowed approximately 1 hour before a meal with a cold or luke-warm [temperature not to exceed body temperature, e.g., 37 °C (98.6 °F)] drink.
Care should be taken not to chew the vaccine capsule. The vaccine capsule should be swallowed as soon after placing in the mouth as possible.
Not all recipients of Vivotif (Typhoid Vaccine Live Oral Ty21a) will be fully protected against typhoid fever. Travelers should take all necessary precautions to avoid contact or ingestion of potentially contaminated food or water.
Several anti-malaria drugs, such as mefloquine, chloroquine and proguanil (not approved for use in US) possess antibacterial activity which may interfere with the immunogenicity of Vivotif. Clinical results (see Warnings – Drug-Interactions) indicate that mefloquine and chloroquine can be administered together with Vivotif. Proguanil should be administered only if 10 days or more have elapsed since the final dose of Vivotif was ingested. Any serious adverse reactions related to the administration of the vaccine should be reported to your health care provider.
You may also report an adverse reaction directly to the Vaccine Adverse Event Reporting System (1–800–822–7967) (20). Your health care provider should inform you of the benefits and risks of the vaccine, the importance of taking all 4 capsules in the correct schedule, and the importance of proper storage temperature of the capsules.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals with Vivotif have not been performed to evaluate carcinogenic potential, mutagenic potential or impairment of fertility.
Pregnancy
Category C
Animal reproduction studies have not been conducted with Vivotif. It is not known whether Vivotif® can cause fetalharm when administered to pregnant women or can affect reproduction capacity. Vivotif® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
There is no data to warrant the use of this product in nursing mothers. It is not known if Vivotif is excreted in human milk.
Pediatric Use
The safety and efficacy of Vivotif has not been established in children under 6 years of age. This product is not indicated for use in children under 6 years of age.
VIVOTIF-B ADVERSE REACTIONS
More than 1.4 million doses of Ty21a have been administered in controlled clinical trials and more than 150 million doses of Vivotif (Typhoid Vaccine Live Oral Ty21a) have been marketed world-wide. Active surveillance for adverse reactions of enteric-coated capsules was performed in a pilot study (21) and in a subgroup of a large field trial (14) involving a total of 483 individuals receiving three vaccine doses. The overall symptom rates from both studies when vaccinated with capsules were combined and shown to be: abdominal pain (6.4%), nausea (5.8%), headache (4.8%), fever (3.3%), diarrhea (2.9%), vomiting (1.5%) and skin rash (1.0%). Only the incidence of nausea occured at a statistically higher frequency in the vaccinated group as compared to the placebo group (14). Administration of vaccine doses more than 5-fold higher than the currently recommended dose caused only mild reactions in an open study involving 155 healthy adult males (16). Post-marketing surveillance has revealed that adverse reactions are infrequent and mild (17). Adverse reactions reported to the manufacturer during 1991–1995, during which time over 60 million doses (capsules) were administered, included: diarrhea (N = 45), abdominal pain (N = 42), nausea (N = 35), fever (N = 34), headache (N = 26), skin rash (N = 26), vomiting (N = 18), or urticaria in the trunk and/or extremities (N = 13). One isolated, non-fatal anaphylactic shock considered to be an allergic reaction to the vaccine was reported.
VIVOTIF-B DOSAGE AND ADMINISTRATION
One capsule is to be swallowed approximately 1 hour before a meal with a cold or luke-warm [temperature not to exceed body temperature, e.g., 37 °C (98.6 °F)] drink on alternate days, e.g., days 1, 3, 5 and 7. Immunization (ingestion of all 4 doses of Vivotif (Typhoid Vaccine Live Oral Ty21a) should be completed at least 1 week prior to potential exposure to S. typhi.
The blister containing the vaccine capsules should be inspected to ensure that the foil seal and capsules are intact.
The vaccine capsule should not be chewed and should be swallowed as soon after placing in the mouth as possible. A complete immunization schedule is the ingestion of 4 vaccine capsules as described above.
Re-immunization
The optimum booster schedule for Vivotif® has not been determined. Efficacy has been shown to persist for at least 5 years. Further, there is no experience with Vivotif as a booster in persons previously immunized with parenteral typhoid vaccine. It is recommended that a re-immunization dose consisting of four vaccine capsules taken on alternate days be given every 5 years under conditions of repeated or continued exposure to typhoid fever (7).
HOW SUPPLIED
A single foil blister contains 4 doses of vaccine in a single package.
Storage
Vivotif (Typhoid Vaccine Live Oral Ty21a) is not stable when exposed to ambient temperatures. Vivotif should therefore be shipped and stored between 2 °C and 8 °C (35.6 °F–46.4 °F). Each package of vaccine shows an expiration date. This expiration date is valid only if the product has been maintained at 2 °C–8 °C (35.6 °F–46.4 °F).
Manufactured by
Berna Biotech Ltd, Berne, Switzerland
US-Licence No. 1632
Distributed by
Berna Products, Crucell Vaccines, Inc., Coral Gables, FL 33146
Repackaged by
Rebel Distributors Corp, Thousand Oaks, CA 91320
References
1. Germanier R., E. Fürer. Isolation and characterisation of Gal E mutant Ty21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J. Infect. Dis. 131: 553–558, 1975.
2. Germanier R., E. Fürer. Characteristics of the attenuated oral vaccine strain S. typhi Ty21a. Develop. Biol. Standard 53: 3–7, 1983.
3. Miller S.I., E.L. Hohmann, D.A. Pegues. Salmonella (including Salmonella typhi). In: Principles and practice of infectious diseases. G.L. Mandell, J.E. Bennett, R. Dolin (ed.) fourth edition, Churchill Livingstone Inc. 2013–2033, 1995.
4. Centers for Disease Control. Summary of notifiable diseases, United States 1995. MMWR 44 (Supplement), 1996.
5. Ryan C.A., N.T. Hargrett-Bean, P.A. Blake. Salmonella typhi infections in the United States, 1975–1984: Increasing role of foreign travel. Rev. Infect. Dis. 11: 1–8, 1989.
6. Taylor D.N., R.A. Pollard, P.A. Blake. Typhoid in the United States and the Risk to the International Traveler. J. Infect Dis. 148: 599–602, 1983.
7. Recommendations of the Advisory Committee on Immunization Practices (ACIP): Typhoid Immunization. MMWR 43 (RR-14), 1994.
8. Ames W.R., M. Robbins. Age and sex as factors in the development of the typhoid carrier state, and a model for estimating carrier prevalence. Am. J. Public Health 33: 221–230, 1943.
9. Wahdan M.H., C. Sérié, Y. Cerisier, S. Sallam, R. Germanier. A controlled field trial of live Salmonella typhi strain Ty21a oral vaccine against typhoid: three-year results. J. Infect. Dis. 145: 292–296, 1982.
10. Black R.E., M.M. Levine, C. Ferreccio, M.L. Clements, C. Lanata, J. Rooney, R. Germanier, Chilean Typhoid Committee. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine 8: 81–84, 1990.
11. Levine M.M., C. Ferreccio, R.E. Black, R. Germanier, Chilean Typhoid Committee. Large-Scale Field Trial of Ty21a Typhoid Vaccine Live Oral Ty21a in Enteric-Coated Capsule Formulation. Lancet 1: 1049–1052, 1987.
12. Levine M.M., C. Ferreccio, R.E. Black, C.O. Tacket, R. Germanier, Chilean Typhoid Committee. Progress in vaccines against typhoid fever. Rev. Infect. Dis. 11 (Supplement 3): S552–S567, 1989.
13. Ferreccio C., M.M. Levine, H. Rodriguez, R. Contreras, Chilean Typhoid Committee. Comparative efficacy of two, three, or four doses of Ty21a live oral typhoid vaccine in enteric-coated capsules: a field trial in endemic area. J. Infect. Dis. 159: 766–769, 1989.
14. Simanjuntak C.H., F.P. Paleologo, N.H. Punjabi, R. Darmowigoto, Soeprawoto, H. Totosudirjo, P. Haryanto, E. Suprijanto, N.D. Witham, S.L. Hoffman. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 338: 1055–1059, 1991.
15. Data on File, Swiss Serum and Vaccine Institute Berne, Switzerland.
16. Gilman R.H., R.B. Hornick, W.E. Woodward, H.L. DuPont, M.J. Snyder, M.M. Levine, J.P. Libonati. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a live oral vaccine. J. Infect. Dis. 136: 717–723, 1977.
17. Cryz S.J. Jr., Post-marketing experience with live oral Ty21a Vaccine. Lancet; 341: 49–50, 1993. Data on File, Swiss Serum and Vaccine Institute Berne, Switzerland.
18. Horowitz H., CA. Carbonaro, Inhibition of the Salmonella typhi oral vaccine strain Ty21a, by mefloquine and chloroquine J. Infect. Dis. 166: 1462–1464, 1992.
19. Kollaritsch H., J.U. Que, C. Kunz, G. Wiedermann, C. Herzog, S.J. Cryz Jr. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with anti-malarial drugs, oral polio vaccine or yellow fever vaccine. J. Infect. Dis. 175: 871–875, 1997.
20. Vaccine Adverse Event Reporting System – United States. MMWR 39: 730–733, 1990.
21. Levine M.M., R.E. Black, C. Ferreccio, M.L. Clements, C. Lanata, J. Rooney, R. Gemanier. The efficacy of attenuated Salmonella typhi oral vaccine strain Ty21a evaluated in controlled field trials. In: Development of Vaccines and Drugs against Diarrhea. 11th Noble Conference, Stockholm, 1985, p. 90–101. J. Holmgren, A. Lindberg and R. Möllby (eds.). Studentlitteratur, Lund, Sweden, 1986.
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
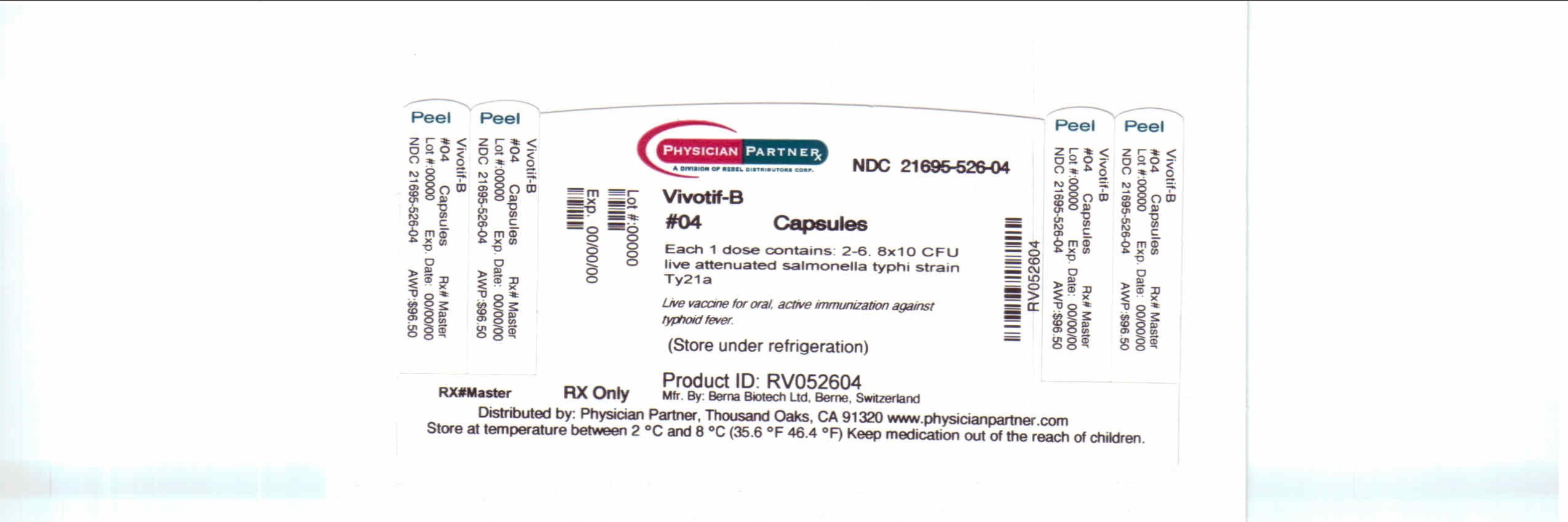
Vivotif-BVivotif-B CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||