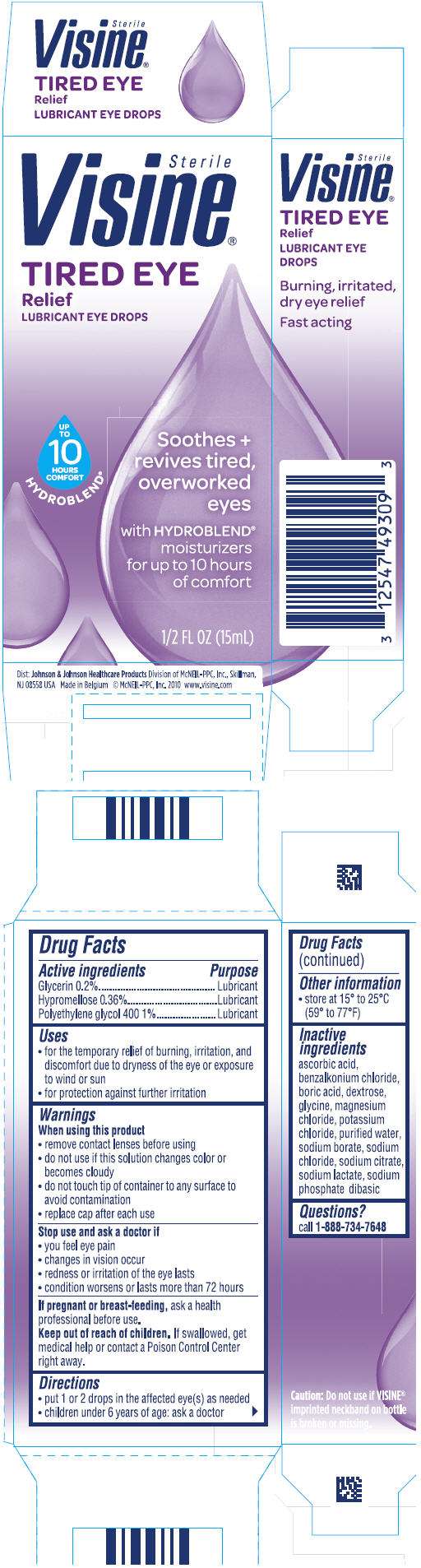
Visine
Johnson & Johnson Healthcare Products, Division of McNEIL-PPC, Inc.
Visine Tired Eye Relief
FULL PRESCRIBING INFORMATION: CONTENTS*
- Visine Uses
- Warnings
- Directions
- Visine Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 15mL Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Glycerin 0.2% | Lubricant |
| Hypromellose 0.36% | Lubricant |
| Polyethylene glycol 400 1% | Lubricant |
Visine Uses
- for the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun
- for protection against further irritation
Warnings
When using this product
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye lasts
- condition worsens or lasts more than 72 hours
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- put 1 or 2 drops in the affected eye(s) as needed
- children under 6 years of age: ask a doctor
Visine Other information
- store at 15° and 25°C (59° to 77°F)
Inactive ingredients
ascorbic acid, benzalkonium chloride, boric acid, dextrose, glycine, magnesium chloride, potassium chloride, purified water, sodium borate, sodium chloride, sodium citrate, sodium lactate, sodium phosphate dibasic
Questions?
Call 1-888-734-7648
Dist: Johnson & Johnson Healthcare Products Division of McNEIL-PPC, Inc., Skillman, NJ 08558 USA
PRINCIPAL DISPLAY PANEL - 15mL Bottle Carton
Sterile
Visine®
TIRED EYE
Relief
LUBRICANT EYE DROPS
UP
TO
10
HOURS COMFORT
HYDROBLEND®
Soothes +
revives tired,
overworked
eyes
with HYDROBLEND®
moisturizers
for up to 10 hours
of comfort
1/2 FL OZ (15mL)
VisineGlycerin, Hypromelloses, and Polyethylene Glycol 400 SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||