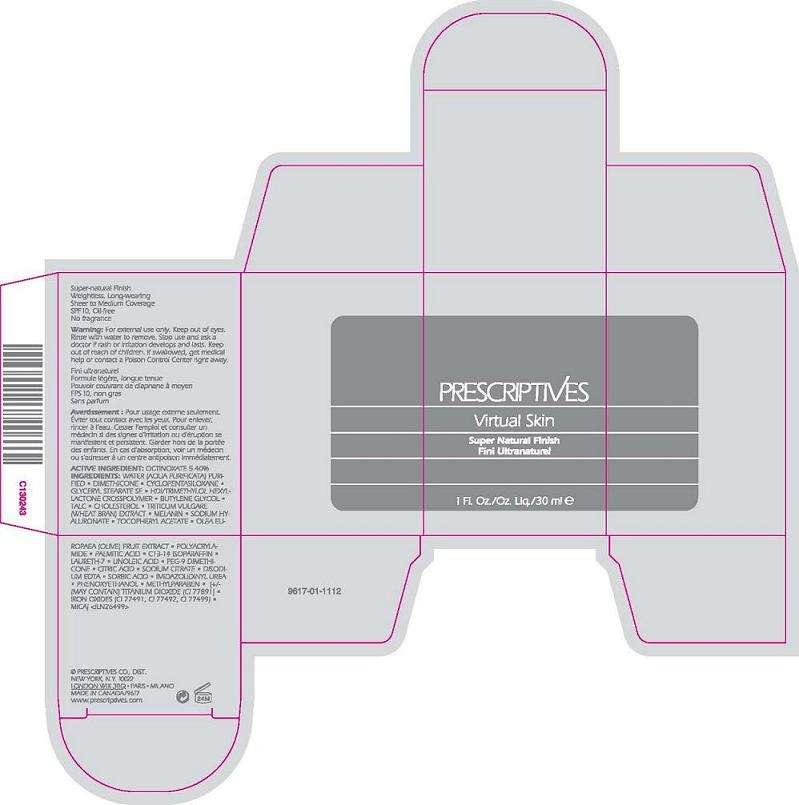
VIRTUAL SKIN
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENT: OCTINOXATE 5.40%
INACTIVE INGREDIENTS: WATER\AQUA\EAU [] DIMETHICONE [] CYCLOPENTASILOXANE [] GLYCERYL STEARATE SE [] HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER [] BUTYLENE GLYCOL [] TALC [] CHOLESTEROL [] TRITICUM VULGARE (WHEAT BRAN) EXTRACT [] MELANIN [] SODIUM HYALURONATE [] TOCOPHERYL ACETATE [] OLEA EUROPAEA (OLIVE) FRUIT EXTRACT [] POLYACRYLAMIDE [] ETHYLHEXYLGLYCERIN [] PALMITIC ACID [] C13-14 ISOPARAFFIN [] CAPRYLYL GLYCOL [] LAURETH-7 [] HEXYLENE GLYCOL [] LINOLEIC ACID [] PEG-9 DIMETHICONE [] SILICA [] CITRIC ACID [] SODIUM CITRATE [] DISODIUM EDTA [] SORBIC ACID [] BENZOIC ACID [] POTASSIUM SORBATE [] PHENOXYETHANOL [] [+/- TITANIUM DIOXIDE (CI 77891) [] IRON OXIDES (CI 77491, CI 77492, CI 77499) [] MICA
WARNINGS: FOR EXTERNAL USE ONLY. KEEP OUT OF EYES. RINSE WITH WATER TO REMOVE. STOP USE AND ASK A DOCTOR IF SKIN RASH OR IRRITATION OCCURS. KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
PRESCRIPTIVES
VIRTUAL SKIN
SUPER NATURAL FINISH
SPF 10
1.0 FL OZ LIQ/ 30 ML
PRESCRIPTIVES CO. DIST
NY NY 10022
VIRTUAL SKINOCTINOXATE LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||