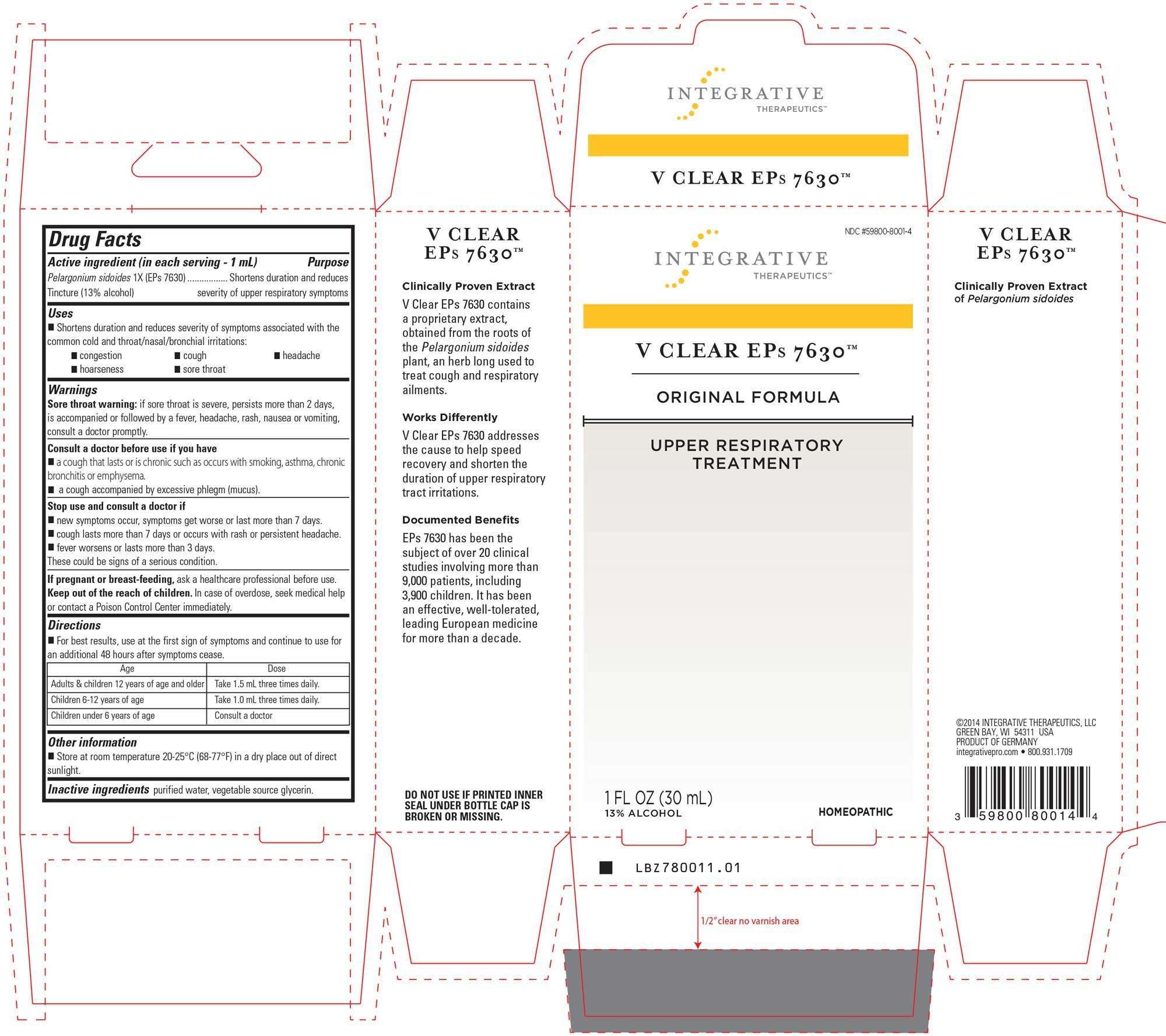
ViraClear EPs 7630 Original
V CLEAR EPs 7630 Original
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient:
PELARGONIUM SIDOIDES
Inactive Ingredients:
PURIFIED WATER
VEGETABLE SOURCE GLYCERIN
ALCOHOL
Dosage and Administration:
Integrative Therapeutics Brand:
Directions:
For best results, use at first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Adults and children 12 years of age and older: Take 1.5 mL 3 times daily.
Children 6-12 years of age: Take 1.0 mL three times daily.
Children under 6 years of age: Consult a doctor
Uses
Indications and Usage:
Shortens duration and reduces severity of symptoms associated with the common cold and throat/nasal/bronchial irritations:
congestion, cough, headache, hoarseness, sore throat.
Purpose
Purpose:
Shortens duration and reduces severity of symptoms associated with the common cold and throat/nasal/bronchial irritations:
congestion, cough, headache, hoarseness, sore throat.
Warnings:
Sore throat warning: if sore throat is severe, persists more than 2 days, is accompanied or followed by a fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Consult a doctor before use if you have a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema.
A cough accompanied by excessive phlegm (mucus).
Stop use and consult a doctor if new symptoms occur, symptoms get worse or last more than 7 days.
Cough lasts more than 7 days or occurs with rash or persistent headache.
Fever worsens or lasts more than 3 days.
These could be signs of a serious condition.
Keep out of Reach of Children:
Keep out of the reach of children.
Ask the Doctor:
Consult a doctor before use if you have a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema.
A cough accompanied by excessive phlegm (mucus).
Stop use and consult a doctor if new symptoms occur, symptoms get worse or last more than 7 days.
Cough lasts more than 7 days or occurs with rash or persistent headache.
Fever worsens or lasts more than 3 days.
These could be signs of a serious condition.
Pregnancy or Breast Feeding:
If pregnant or breast-feeding, ask a healthcare professional before use.
In case of overdose, seek medical help or contact a Poison Control Center immediately.
780011_01 V Clear EPS 7630
780011_01.jpg
ViraClear EPs 7630 OriginalPelargonium sidoides LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||