Venlafaxine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- VENLAFAXINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of venlafaxine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Venlafaxine hydrochloride tablets are not approved for use in pediatric patients (seeWARNINGS: Clinical Worsening and Suicide Risk,PRECAUTIONS: Information for Patients, andPRECAUTIONS: Pediatric Use).
VENLAFAXINE HYDROCHLORIDE DESCRIPTION
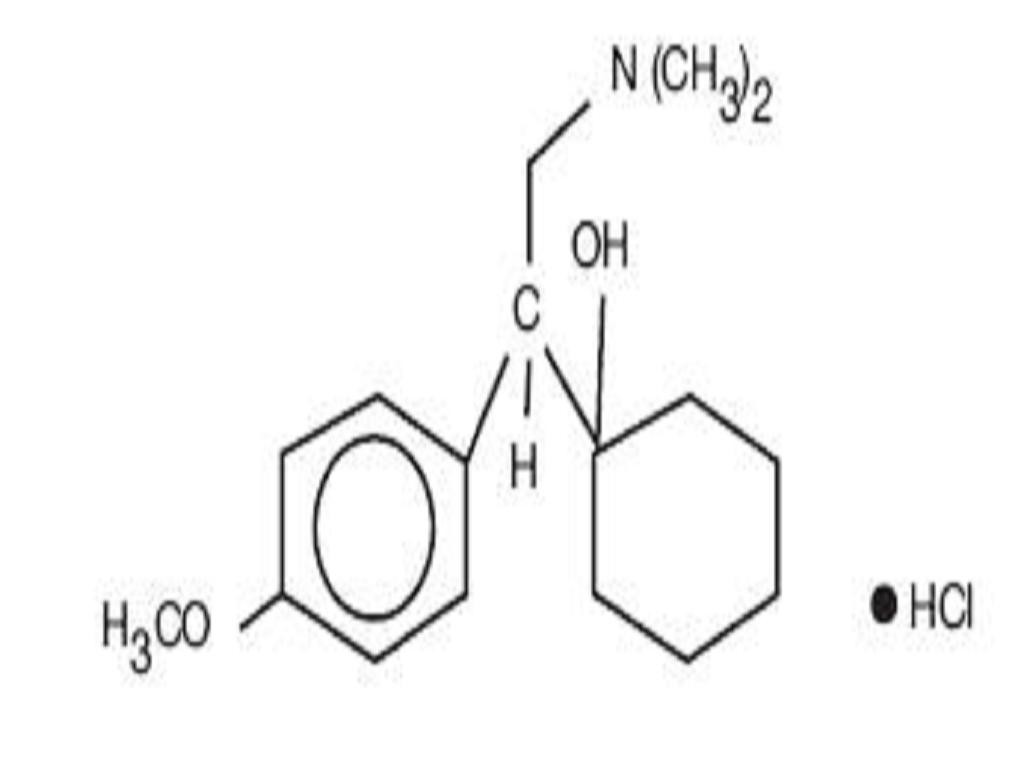
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Age and Gender
DOSAGE AND ADMINISTRATION
Liver Disease
DOSAGE AND ADMINISTRATION
Renal Disease
DOSAGE AND ADMINISTRATION
CLINICAL TRIALS
INDICATIONS & USAGE
CLINICAL TRIALS
CLINICAL TRIALS
VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
DOSAGE AND ADMINISTRATION
WARNINGS
Clinical Worsening and Suicide RiskAll patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
PRECAUTIONSDOSAGE AND ADMINISTRATION: Discontinuation of Treatment with Venlafaxine Hydrochloride Tablets
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
PRECAUTIONS: Drug Interactions
CONTRAINDICATIONS
PRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
Sustained Hypertension
Mydriasis
PRECAUTIONS: Information for Patients
PRECAUTIONS
General
Discontinuation of Treatment with Venlafaxine Hydrochloride Tablets
DOSAGE AND ADMINISTRATION
Anxiety and Insomnia
Changes in Weight
Pediatric Patients
PRECAUTIONS, General, Changes in Appetite
Changes in Height
Changes in Appetite
Pediatric Patients
Activation of Mania/Hypomania
Hyponatremia
PRECAUTIONS, Geriatric Use
Seizures
Abnormal Bleeding
Serum Cholesterol Elevation
ADVERSE REACTIONS, Laboratory Changes
Interstitial Lung Disease and Eosinophilic Pneumonia
Use in Patients with Concomitant Illness
DOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
Interference with Cognitive and Motor Performance
Pregnancy
Nursing
Mydriasis
WARNINGS
Concomitant Medication
WARNINGS, Serotonin SyndromePRECAUTIONS, Drug Interactions, CNS-Active Drugs
PRECAUTIONS, Abnormal Bleeding
Alcohol
Allergic Reactions
LABORATORY TESTS
DRUG INTERACTIONS
Alcohol
Cimetidine
Diazepam
Haloperidol
Lithium
CNS-Active Drugs,
Drugs Highly Bound to Plasma Protein
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Drugs that Inhibit Cytochrome P450 Isoenzymes
Ketoconazole
CYP3A4 Inhibitors
Drugs Metabolized by Cytochrome P450 Isoenzymes
Imipramine
Metoprolol
WARNINGS
Risperidone
CYP3A4
Indinavir
CYP1A2
CYP2C9
CYP2C19
Diazepam
Monoamine Oxidase Inhibitors
CONTRAINDICATIONS
CNS-Active Drugs
Serotonergic Drugs
WARNINGS, Serotonin SyndromeWARNINGS, Serotonin SyndromeWARNINGS, Serotonin Syndrome
Triptans
WARNINGS, Serotonin Syndrome
Electroconvulsive Therapy
Postmarketing Spontaneous Drug Interaction Reports
ADVERSE REACTIONS, Postmarketing Reports
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenicity
Impairment of Fertility
PREGNANCY
Teratogenic EffectsPregnancy Category CNon-teratogenic Effects
PRECAUTIONS-Drug Interactions- CNS-Active DrugsDOSAGE AND ADMINISTRATION
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGS, Clinical Worsening and Suicide RiskPRECAUTIONS , General,Changes in HeightChanges in Weight
WARNINGS, Sustained HypertensionPRECAUTIONS, General, Serum Cholesterol Elevation
GERIATRIC USE
PRECAUTIONS, HyponatremiaCLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
Associated with Discontinuation of Treatment**
Incidence in Controlled Trials
Commonly Observed Adverse Events in Controlled Clinical Trials
Adverse Events Occurring at an Incidence of 1% or More Among Venlafaxine Hydrochloride Tablets -Treated Patients
*
*
Dose Dependency of Adverse Events
Adaptation to Certain Adverse Events
Vital Sign Changes
WARNINGS
Laboratory Changes
PRECAUTIONS, General-Serum Cholesterol Elevation
ECG Changes
PRECAUTIONS, General, Use in Patients with Concomitant Illness
Other Events Observed During the Premarketing Evaluation of Venlafaxine
Postmarketing Reports
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
DOSAGE AND ADMINISTRATION
OVERDOSAGE
Human ExperienceManagement of Overdosage
DOSAGE & ADMINISTRATION
Initial TreatmentPRECAUTIONS, General, Use in Patients with Concomitant Illness
Special Populations
Treatment of Pregnant Women During the Third Trimester
PRECAUTIONS
Dosage for Patients with Hepatic Impairment
CLINICAL PHARMACOLOGY
Dosage for Patients with Renal Impairment
CLINICAL PHARMACOLOGY
Dosage for Elderly Patients
Maintenance Treatment
CLINICAL TRIALS
Discontinuing Venlafaxine Hydrochloride Tablets
PRECAUTIONS
SWITCHING PATIENTS TO OR FROM A MONOAMINE OXIDASE INHIBITOR
CONTRAINDICATIONS
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Venlafaxine Hydrochloride TabletsRx Only
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family member's, healthcare provider about:
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
What else do I need to know about antidepressant medicines?
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your child's healthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Venlafaxine HydrochlorideVenlafaxine Hydrochloride TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!