Venlafaxine Hydrochloride
St. Marys Medical Park Pharmacy
Venlafaxine Hydrochloride
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of venlafaxine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Venlafaxine hydrochloride tablets are not approved for use in pediatric patients (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
DESCRIPTION
Venlafaxine hydrochloride is a structurally novel antidepressant for oral administration. It is designated (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[α-[(dimethyl-amino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the molecular formula of C17H27NO2 HCl. Its molecular weight is 313.87. The structural formula is shown below.
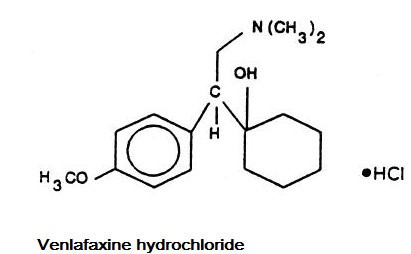
Venlafaxine hydrochloride is a white crystalline powder. It is freely soluble in water and dilute hydrochloric acid, soluble in ethanol and chloroform and insoluble in ether.
Each venlafaxine hydrochloride tablet intended for oral administration contains venlafaxine hydrochloride equivalent to 25 mg or 37.5 mg or 50 mg or 75 mg or 100 mg of venlafaxine. In addition, each tablet contains the following inactive ingredients: ferric oxide yellow, ferric oxide red, lactose monohydrate, magnesium stearate, microcrystalline cellulose and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Pharmacodynamics
The mechanism of the antidepressant action of venlafaxine in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Preclinical studies have shown that venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Venlafaxine and ODV have no significant affinity for muscarinic, histaminergic, or α-1 adrenergic receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Venlafaxine and ODV do not possess monoamine oxidase (MAO) inhibitory activity.
Pharmacokinetics
Venlafaxine is well absorbed and extensively metabolized in the liver. O-desmethylvenlafaxine (ODV) is the only major active metabolite. On the basis of mass balance studies, at least 92% of a single dose of venlafaxine is absorbed. Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as either unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is the primary route of excretion. The relative bioavailability of venlafaxine from a tablet was 100% when compared to an oral solution. Food has no significant effect on the absorption of venlafaxine or on the formation of ODV.
The degree of binding of venlafaxine to human plasma is 27% ± 2% at concentrations ranging from 2.5 to 2215 ng/mL. The degree of ODV binding to human plasma is 30% ± 12% at concentrations ranging from 100 to 500 ng/mL. Protein-binding-induced drug interactions with venlafaxine are not expected.
Steady-state concentrations of both venlafaxine and ODV in plasma were attained within 3 days of multiple-dose therapy. Venlafaxine and ODV exhibited linear kinetics over the dose range of 75 to 450 mg total dose per day (administered on a q8h schedule). Plasma clearance, elimination half-life and steady-state volume of distribution were unaltered for both venlafaxine and ODV after multiple-dosing. Mean ± SD steady-state plasma clearance of venlafaxine and ODV is 1.3 ± 0.6 and 0.4 ± 0.2 L/h/kg, respectively; elimination half-life is 5 ± 2 and 11±2 hours, respectively; and steady-state volume of distribution is 7.5 ± 3.7 L/kg and 5.7 ± 1.8 L/kg, respectively. When equal daily doses of venlafaxine were administered as either b.i.d. or t.i.d. regimens, the drug exposure (AUC) and fluctuation in plasma levels of venlafaxine and ODV were comparable following both regimens.
Age and Gender
A pharmacokinetic analysis of 404 venlafaxine-treated patients from two studies involving both b.i.d. and t.i.d. regimens showed that dose-normalized trough plasma levels of either venlafaxine or ODV were unaltered due to age or gender differences. Dosage adjustment based upon the age or gender of a patient is generally not necessary (see DOSAGE AND ADMINISTRATION).
Liver Disease
In 9 subjects with hepatic cirrhosis, the pharmacokinetic disposition of both venlafaxine and ODV was significantly altered after oral administration of venlafaxine. Venlafaxine elimination half-life was prolonged by about 30%, and clearance decreased by about 50% in cirrhotic subjects compared to normal subjects. ODV elimination half-life was prolonged by about 60% and clearance decreased by about 30% in cirrhotic subjects compared to normal subjects. A large degree of intersubject variability was noted. Three patients with more severe cirrhosis had a more substantial decrease in venlafaxine clearance (about 90%) compared to normal subjects.
In a second study, venlafaxine was administered orally and intravenously in normal (n = 21) subjects, and in Child-Pugh A (n = 8) and Child-Pugh B (n = 11) subjects (mildly and moderately impaired, respectively). Venlafaxine oral bioavailability was increased 2-3 fold, oral elimination half-life was approximately twice as long and oral clearance was reduced by more than half, compared to normal subjects. In hepatically impaired subjects, ODV oral elimination half-life was prolonged by about 40%, while oral clearance for ODV was similar to that for normal subjects. A large degree of intersubject variability was noted.
Dosage adjustment is necessary in these hepatically impaired patients (see DOSAGE AND ADMINISTRATION).
Renal Disease
In a renal impairment study, venlafaxine elimination half-life after oral administration was prolonged by about 50% and clearance was reduced by about 24% in renally impaired patients (GFR = 10-70 mL/min), compared to normal subjects. In dialysis patients, venlafaxine elimination half-life was prolonged by about 180% and clearance was reduced by about 57% compared to normal subjects. Similarly, ODV elimination half-life was prolonged by about 40% although clearance was unchanged in patients with renal impairment (GFR = 10-70 mL/min) compared to normal subjects. In dialysis patients, ODV elimination half-life was prolonged by about 142% and clearance was reduced by about 56%, compared to normal subjects. A large degree of intersubject variability was noted.
Dosage adjustment is necessary in these patients (see DOSAGE AND ADMINISTRATION).
CLINICAL TRIALS
The efficacy of venlafaxine hydrochloride tablets as a treatment for major depressive disorder was established in 5 placebo-controlled, short-term trials. Four of these were 6-week trials in adult outpatients meeting DSM-III or DSM-III-R criteria for major depression: two involving dose titration with venlafaxine hydrochloride tablets in a range of 75 to 225 mg/day (t.i.d. schedule), the third involving fixed venlafaxine hydrochloride tablets doses of 75, 225, and 375 mg/day (t.i.d. schedule), and the fourth involving doses of 25, 75, and 200 mg/day (b.i.d. schedule). The fifth was a 4-week study of adult inpatients meeting DSM-III-R criteria for major depression with melancholia whose venlafaxine hydrochloride tablets doses were titrated in a range of 150 to 375 mg/day (t.i.d. schedule). In these 5 studies, venlafaxine hydrochloride tablets were shown to be significantly superior to placebo on at least 2 of the following 3 measures: Hamilton Depression Rating Scale (total score), Hamilton depressed mood item, and Clinical Global Impression-Severity of Illness rating. Doses from 75 to 225 mg/day were superior to placebo in outpatient studies and a mean dose of about 350 mg/day was effective in inpatients. Data from the 2 fixed-dose outpatient studies were suggestive of a dose-response relationship in the range of 75 to 225 mg/day. There was no suggestion of increased response with doses greater than 225 mg/day.
While there were no efficacy studies focusing specifically on an elderly population, elderly patients were included among the patients studied. Overall, approximately 2/3 of all patients in these trials were women. Exploratory analyses for age and gender effects on outcome did not suggest any differential responsiveness on the basis of age or sex.
In one longer-term study, adult outpatients meeting DSM-IV criteria for major depressive disorder who had responded during an 8-week open trial on venlafaxine hydrochloride extended-release capsules (75, 150, or 225 mg, qAM) were randomized to continuation of their same venlafaxine hydrochloride extended-release capsules dose or to placebo, for up to 26 weeks of observation for relapse. Response during the open phase was defined as a CGI Severity of Illness item score of ≤ 3 and a HAM-D-21 total score of ≤ 10 at the day 56 evaluation. Relapse during the double-blind phase was defined as follows: (1) a reappearance of major depressive disorder as defined by DSM-IV criteria and a CGI Severity of Illness item score of ≥ 4 (moderately ill), (2) 2 consecutive CGI Severity of Illness item scores of ≥ 4, or (3) a final CGI Severity of Illness item score of ≥ 4 for any patient who withdrew from the study for any reason. Patients receiving continued venlafaxine hydrochloride extended-release capsules treatment experienced significantly lower relapse rates over the subsequent 26 weeks compared with those receiving placebo.
In a second longer-term trial, adult outpatients meeting DSM-III-R criteria for major depression, recurrent type, who had responded (HAM-D-21 total score ≤ 12 at the day 56 evaluation) and continued to be improved [defined as the following criteria being met for days 56 through 180: (1) no HAM-D-21 total score ≥ 20; (2) no more than 2 HAM-D-21 total scores > 10; and (3) no single CGI Severity of Illness item score ≥ 4 (moderately ill)] during an initial 26 weeks of treatment on venlafaxine hydrochloride tablets (100 to 200 mg/day, on a b.i.d. schedule) were randomized to continuation of their same venlafaxine hydrochloride tablets dose or to placebo. The follow-up period to observe patients for relapse, defined as a CGI Severity of Illness item score ≥ 4, was for up to 52 weeks. Patients receiving continued venlafaxine hydrochloride tablets treatment experienced significantly lower relapse rates over the subsequent 52 weeks compared with those receiving placebo.
Uses
INDICATIONS AND USAGE
Venlafaxine hydrochloride tablets are indicated for the treatment of major depressive disorder.
The efficacy of venlafaxine hydrochloride tablets in the treatment of major depressive disorder was established in 6-week controlled trials of adult outpatients whose diagnoses corresponded most closely to the DSM-III or DSM-III-R category of major depression and in a 4-week controlled trial of inpatients meeting diagnostic criteria for major depression with melancholia (see CLINICAL TRIALS).
A major depressive episode implies a prominent and relatively persistent depressed or dysphoric mood that usually interferes with daily functioning (nearly every day for at least 2 weeks); it should include at least 4 of the following 8 symptoms: change in appetite, change in sleep, psychomotor agitation or retardation, loss of interest in usual activities or decrease in sexual drive, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, and a suicide attempt or suicidal ideation.
The efficacy of venlafaxine hydrochloride extended-release capsules in maintaining an antidepressant response for up to 26 weeks following 8 weeks of acute treatment was demonstrated in a placebo-controlled trial. The efficacy of venlafaxine hydrochloride tablets in maintaining an antidepressant response in patients with recurrent depression who had responded and continued to be improved during an initial 26 weeks of treatment and were then followed for a period of up to 52 weeks was demonstrated in a second placebo-controlled trial (see CLINICAL TRIALS). Nevertheless, the physician who elects to use venlafaxine hydrochloride tablets/venlafaxine hydrochloride extended-release capsules for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
CONTRAINDICATIONS
Hypersensitivity to venlafaxine hydrochloride or to any excipients in the formulation.
Venlafaxine hydrochloride Tablets must not be used concomitantly in patients taking MAOIs or in patients who have taken MAOIs within the preceding 14 days due to the risk of serious, sometimes fatal, drug interactions with SNRI or SSRI treatment or with other serotonergic drugs. These interactions have been associated with symptoms that include tremor, myoclonus, diaphoresis, nausea, vomiting, flushing, dizziness, hyperthermia with features resembling neuroleptic malignant syndrome, seizures, rigidity, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. Based on the half-life of venlafaxine, at least 7 days should be allowed after stopping venlafaxine hydrochloride tablets before starting an MAOI (see DOSAGE AND ADMINISTRATION).
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Age Range |
Drug-Placebo Difference in N umber of Cases of Suicidality per 1000 Patients Treated |
|
|
Increases Compared to Placebo |
| less than 18 |
14 additional cases |
| 18-24 |
5 additional cases |
|
|
Decreases Compared to Placebo |
| 25-64 |
1 fewer case |
| greaterthan or equals to 65 |
6 fewer cases |
| Treatment Group |
Incidence of Sustained Elevation in SDBP |
| Venlafaxine |
|
| Lessthan 100 mg/day |
3% |
| 101-200 mg/day |
5% |
| 201-300 mg/day |
7% |
| greater than 300 mg/day |
13% |
| Placebo |
2% |
PRECAUTIONS
General
Discontinuation of Treatment with Venlafaxine Hydrochloride Tablets
Discontinuation symptoms have been systematically evaluated in patients taking venlafaxine, to include prospective analyses of clinical trials in Generalized Anxiety Disorder and retrospective surveys of trials in major depressive disorder. Abrupt discontinuation or dose reduction of venlafaxine at various doses has been found to be associated with the appearance of new symptoms, the frequency of which increased with increased dose level and with longer duration of treatment. Reported symptoms include agitation, anorexia, anxiety, confusion, impaired coordination and balance, diarrhea, dizziness, dry mouth, dysphoric mood, fasciculation, fatigue, flu-like symptoms, headaches, hypomania, insomnia, nausea, nervousness, nightmares, sensory disturbances (including shock-like electrical sensations), somnolence, sweating, tremor, vertigo, and vomiting.
During marketing of venlafaxine hydrochloride tablets, other SNRIs (Serotonin and Norepinephrine Reuptake Inhibitors), and SSRIs (Selective Serotonin Reuptake Inhibitors), there have been spontaneous reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g. paresthesias such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
Patients should be monitored for these symptoms when discontinuing treatment with venlafaxine hydrochloride tablets. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate (see DOSAGE AND ADMINISTRATION).
Anxiety and Insomnia
Treatment-emergent anxiety, nervousness, and insomnia were more commonly reported for venlafaxine-treated patients compared to placebo-treated patients in a pooled analysis of short-term, double-blind, placebo-controlled depression studies:
| Symptom |
Venlafaxine n = 1033 |
Placebo n = 609 |
| Anxiety |
6% |
3% |
| Nervousness |
13% |
6% |
| Insomnia |
18% |
10% |
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Venlafaxine was given by oral gavage to mice for 18 months at doses up to 120 mg/kg per day, which was 16 times, on a mg/kg basis, and 1.7 times on a mg/m2 basis, the maximum recommended human dose. Venlafaxine was also given to rats by oral gavage for 24 months at doses up to 120 mg/kg per day. In rats receiving the 120 mg/kg dose, plasma levels of venlafaxine were 1 times (male rats) and 6 times (female rats) the plasma levels of patients receiving the maximum recommended human dose. Plasma levels of the O-desmethyl metabolite were lower in rats than in patients receiving the maximum recommended dose. Tumors were not increased by venlafaxine treatment in mice or rats.
Mutagenicity
Venlafaxine and the major human metabolite, O-desmethylvenlafaxine (ODV), were not mutagenic in the Ames reverse mutation assay in Salmonella bacteria or the CHO/HGPRT mammalian cell forward gene mutation assay. Venlafaxine was also not mutagenic in the in vitro BALB/c-3T3 mouse cell transformation assay, the sister chromatid exchange assay in cultured CHO cells, or the in vivo chromosomal aberration assay in rat bone marrow. ODV was not mutagenic in the in vitro CHO cell chromosomal aberration assay. There was a clastogenic response in the in vivo chromosomal aberration assay in rat bone marrow in male rats receiving 200 times, on a mg/kg basis, or 50 times, on a mg/m2 basis, the maximum human daily dose. The no effect dose was 67 times (mg/kg) or 17 times (mg/m2) the human dose.
Impairment of Fertility
Reproduction and fertility studies in rats showed no effects on male or female fertility at oral doses of up to 8 times the maximum recommended human daily dose on a mg/kg basis, or up to 2 times on a mg/ m2 basis.
Pregnancy
Teratogenic Effects—Pregnancy Category C
Venlafaxine did not cause malformations in offspring of rats or rabbits given doses up to 11 times (rat) or 12 times (rabbit) the maximum recommended human daily dose on a mg/kg basis, or 2.5 times (rat) and 4 times (rabbit) the human daily dose on a mg/m2 basis. However, in rats, there was a decrease in pup weight, an increase in stillborn pups, and an increase in pup deaths during the first 5 days of lactation, when dosing began during pregnancy and continued until weaning. The cause of these deaths is not known. These effects occurred at 10 times (mg/kg) or 2.5 times (mg/m2) the maximum human daily dose. The no effect dose for rat pup mortality was 1.4 times the human dose on a mg/kg basis or 0.25 times the human dose on a mg/m2 basis. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Non-teratogenic Effects
Neonates exposed to venlafaxine hydrochloride tablets, other SNRIs (Serotonin and Norepinephrine Reuptake Inhibitors), or SSRIs (Selective Serotonin Reuptake Inhibitors), late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome (see PRECAUTIONS-Drug Interactions- CNS-Active Drugs). When treating a pregnant woman with venlafaxine hydrochloride tablets during the third trimester, the physician should carefully consider the potential risks and benefits of treatment (see DOSAGE AND ADMINISTRATION).
Labor and Delivery
The effect of venlafaxine hydrochloride tablets on labor and delivery in humans is unknown.
Nursing Mothers
Venlafaxine and ODV have been reported to be excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from venlafaxine hydrochloride tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS, Clinical Worsening and Suicide Risk). Two placebo-controlled trials in 766 pediatric patients with MDD and two placebo-controlled trials in 793 pediatric patients with GAD have been conducted with venlafaxine hydrochloride extended-release capsules, and the data were not sufficient to support a claim for use in pediatric patients.
Anyone considering the use of venlafaxine hydrochloride tablets in a child or adolescent must balance the potential risks with the clinical need.
Although no studies have been designed to primarily assess venlafaxine hydrochloride extended-release capsule’s impact on the growth, development, and maturation of children and adolescents, the studies that have been done suggest that venlafaxine hydrochloride extended-release capsules may adversely affect weight and height (see PRECAUTIONS , General,Changes in Height and Changes in Weight). Should the decision be made to treat a pediatric patient with venlafaxine hydrochloride tablets, regular monitoring of weight and height is recommended during treatment, particularly if it is to be continued long term. The safety of venlafaxine hydrochloride extended-release capsules treatment for pediatric patients has not been systematically assessed for chronic treatment longer than six months in duration.
In the studies conducted in pediatric patients (ages 6-17), the occurrence of blood pressure and cholesterol increases considered to be clinically relevant in pediatric patients was similar to that observed in adult patients. Consequently, the precautions for adults apply to pediatric patients (see WARNINGS, Sustained Hypertension, and PRECAUTIONS, General, Serum Cholesterol Elevation).
Geriatric Use
Of the 2,897 patients in Phase 2 and Phase 3 depression studies with venlafaxine hydrochloride tablets, 12% (357) were 65 years of age or over. No overall differences in effectiveness or safety were observed between these patients and younger patients, and other reported clinical experience generally has not identified differences in response between the elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out. SSRIs and SNRIs, including venlafaxine, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event (see PRECAUTIONS, Hyponatremia).
The pharmacokinetics of venlafaxine and ODV are not substantially altered in the elderly (see CLINICAL PHARMACOLOGY). No dose adjustment is recommended for the elderly on the basis of age alone, although other clinical circumstances, some of which may be more common in the elderly, such as renal or hepatic impairment, may warrant a dose reduction (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Associated with Discontinuation of Treatment
Nineteen percent (537/2897) of venlafaxine patients in Phase 2 and Phase 3 depression studies discontinued treatment due to an adverse event. The more common events (≥ 1%) associated with discontinuation and considered to be drug-related (i.e., those events associated with dropout at a rate approximately twice or greater for venlafaxine compared to placebo) included:
| CNS |
Venlafaxine |
Placebo |
| Somnolence |
3% |
1% |
| Insomnia |
3% | 1% |
| Dizziness |
3% | - |
| Nervousness |
2% | - |
| Dry mouth |
2% | - |
| Anxiety |
2% | 1% |
| Gastrointestinal |
|
|
| Nausea |
6% |
|
| Urogenital |
|
|
| Abnormal |
3% | - |
| ejaculation* |
|
|
| Other |
|
|
| Headache |
3% | 1% |
| Asthenia |
2% | - |
| Sweating |
2% | - |
| Body System |
Preferred term |
Venlafaxine Hydrochloride Tablets (n=1033) |
Placebo (n=609) |
| Body as a Whole |
Headache |
25% |
24% |
|
|
Asthenia |
12% | 6% |
|
|
Infection |
6% | 5% |
|
|
Chills |
3% | - |
|
|
Chest pain |
2% | 1% |
|
|
Trauma |
2% | 1% |
| Cardiovascular |
Vasodilatation |
4% | 3% |
|
|
Increased blood pressure/hypertension | 2% |
- |
|
|
Tachycardia |
2% | - |
|
|
Postural hypotension |
1% | - |
| Dermatological |
Sweating |
12% | 3% |
|
|
Rash |
3% | 2% |
|
|
Pruritus |
1% | - |
| Gastrointestinal |
Nausea |
37% | 11% |
|
|
Constipation |
15% | 7% |
|
|
Anorexia |
11% |
2% |
|
|
Diarrhea |
8% | 7% |
|
|
Vomiting |
6% | 2% |
|
|
Dyspepsia |
5% | 4% |
|
|
Flatulence |
3% | 2% |
| Metabolic |
Weight loss |
1% | - |
| Nervous System |
Somnolence |
23% | 9% |
|
|
Dry mouth |
22% | 11% |
|
|
Dizziness |
19% | 7% |
|
|
Insomnia |
18% | 10% |
|
|
Nervousness |
13% | 6% |
|
|
Anxiety |
6% | 3% |
|
|
Tremor |
5% | 1% |
|
|
Abnormal dreams |
4% | 3% |
|
|
Hypertonia |
3% | 2% |
|
|
Paresthesia |
3% | 2% |
|
|
Libido decreased |
2% | - |
|
|
Agitation |
2% | - |
|
|
Confusion |
2% | 1% |
|
|
Thinking abnormal |
2% | 1% |
|
|
Depersonalization |
1% | - |
|
|
Depression |
1% | - |
|
|
Urinary retention |
1% | - |
|
|
Twitching |
1% | - |
| Respiration |
Yawn |
3% | - |
| Special Senses |
Blurred vision |
6% | 2% |
|
|
Taste perversion |
2% | - |
|
|
Tinnitus |
2% | - |
|
|
Mydriasis |
2% | - |
|
|
Abnormal ejaculation/Orgasm |
12% 2
|
- 2
|
|
|
Impotence |
6% 2
|
- 2
|
|
|
Urinary frequency |
3% | 2% |
|
|
Urination impaired |
2% | - |
|
|
Orgasm disturbance |
2% 3
|
- 3
|
2
3
| Body System / Preferred Term |
Venlafaxine Hydrochloride Tablets (mg/day) |
Venlafaxine Hydrochloride Tablets (mg/day) |
Venlafaxine Hydrochloride Tablets (mg/day) |
Venlafaxine Hydrochloride Tablets (mg/day) |
|
|
Placebo (n=92) |
75 (n=89) |
225 (n=89) |
375 (n=88) |
| Body as a Whole |
|
|
|
|
| Abdominal pain |
3.3% | 3.4% |
2.2% | 8.0% |
| Asthenia |
3.3% | 16.9% | 14.6% | 14.8% |
| Chills |
1.1% |
2.2% | 5.6% | 6.8% |
| Infection |
2.2% |
2.2% | 5.6% | 2.3% |
| Cardiovascular System |
|
|
|
|
| Hypertension |
1.1% |
1.1% | 2.2% | 4.5% |
| Vasodilatation |
0.0% |
4.5% | 5.6% | 2.3% |
| Digestive System |
|
|
|
|
| Anorexia |
2.2% | 14.6% | 13.5% | 17.0% |
| Dyspepsia |
2.2% | 6.7% | 6.7% | 4.5% |
| Nausea |
14.1% |
32.6% | 38.2% | 58.0% |
| Vomiting |
1.1% |
7.9% | 3.4% | 6.8% |
| Nervous System |
|
|
|
|
| AGitation |
0.0% |
1.1% | 2.2% | 4.5% |
| Anxiety |
4.3% |
11.2% | 4.5% | 2.3% |
| Dizziness |
4.3% |
19.1% | 22.5% | 23.9% |
| Insomnia |
9.8% |
22.5% | 20.2% | 13.6% |
| Libido decreased |
1.1% | 2.2% | 1.1% | 5.7% |
| Nervousness |
4.3% | 21.3% | 13.5% | 12.5% |
| Somnolence |
4.3% | 16.9% | 18.0% | 26.1% |
| Tremor |
0.0% | 1.1% | 2.2% | 10.2% |
| Respiratory System |
|
|
|
|
| Yawn |
0.0% | 4.5% | 5.6% | 8.0% |
| Skin and Appendages |
|
|
|
|
| Sweating |
5.4% |
6.7% |
12.4% | 19.3% |
| Special Senses |
|
|
|
|
| Abnormality of accommodation |
0.0% | 9.1% | 7.9% | 5.6% |
| Urogenital System |
|
|
|
|
| Abnormal ejaculation/orgasm | 0.0% | 4.5% | 2.2% | 12.5% |
| Impotence |
0.0% | 5.8% | 2.1% | 3.6% |
| (Number of men) |
(n=63) |
(n=52) |
(n=48) |
(n=56) |
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Venlafaxine hydrochloride tablets are not a controlled substance.
Physical and Psychological Dependence
In vitro studies revealed that venlafaxine has virtually no affinity for opiate, benzodiazepine, phencyclidine (PCP), or N-methyl-D-aspartic acid (NMDA) receptors.
Venlafaxine was not found to have any significant CNS stimulant activity in rodents. In primate drug discrimination studies, venlafaxine showed no significant stimulant or depressant abuse liability.
Discontinuation effects have been reported in patients receiving venlafaxine (see DOSAGE AND ADMINISTRATION).
While venlafaxine hydrochloride tablets have not been systematically studied in clinical trials for its potential for abuse, there was no indication of drug-seeking behavior in the clinical trials. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of venlafaxine hydrochloride tablets (e.g., development of tolerance, incrementation of dose, drug-seeking behavior).
OVERDOSAGE
Human Experience
There were 14 reports of acute overdose with venlafaxine hydrochloride tablets, either alone or in combination with other drugs and/or alcohol, among the patients included in the premarketing evaluation. The majority of the reports involved ingestions in which the total dose of venlafaxine hydrochloride tablets taken was estimated to be no more than several-fold higher than the usual therapeutic dose. The 3 patients who took the highest doses were estimated to have ingested approximately 6.75 g, 2.75 g, and 2.5 g. The resultant peak plasma levels of venlafaxine for the latter 2 patients were 6.24 and 2.35 µg/mL, respectively, and the peak plasma levels of O-desmethylvenlafaxine were 3.37 and 1.30 µg/mL, respectively. Plasma venlafaxine levels were not obtained for the patient who ingested 6.75 g of venlafaxine. All 14 patients recovered without sequelae. Most patients reported no symptoms. Among the remaining patients, somnolence was the most commonly reported symptom. The patient who ingested 2.75 g of venlafaxine was observed to have 2 generalized convulsions and a prolongation of QTc to 500 msec, compared with 405 msec at baseline. Mild sinus tachycardia was reported in 2 of the other patients.
In postmarketing experience, overdose with venlafaxine has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported events in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
Published retrospective studies report that venlafaxine overdosage may be associated with an increased risk of fatal outcomes compared to that observed with SSRI antidepressant products, but lower than that for tricyclic antidepressants. Epidemiological studies have shown that venlafaxine-treated patients have a higher pre-existing burden of suicide risk factors than SSRI-treated patients. The extent to which the finding of an increased risk of fatal outcomes can be attributed to the toxicity of venlafaxine in overdosage as opposed to some characteristic(s) of venlafaxine-treated patients is not clear. Prescriptions for venlafaxine hydrochloride tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Management of Overdosage
Treatment should consist of those general measures employed in the management of overdosage with any antidepressant.
Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients. Activated charcoal should be administered. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion and exchange transfusion are unlikely to be of benefit. No specific antidotes for venlafaxine are known.
In managing overdosage, consider the possibility of multiple drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians’ Desk Reference (PDR).
DOSAGE AND ADMINISTRATION
Initial Treatment
The recommended starting dose for venlafaxine hydrochloride tablets is 75 mg/day, administered in two or three divided doses, taken with food. Depending on tolerability and the need for further clinical effect, the dose may be increased to 150 mg/day. If needed, the dose should be further increased up to 225 mg/day. When increasing the dose, increments of up to 75 mg/day should be made at intervals of no less than 4 days. In outpatient settings there was no evidence of usefulness of doses greater than 225 mg/day for moderately depressed patients, but more severely depressed inpatients responded to a mean dose of 350 mg/day. Certain patients, including more severely depressed patients, may therefore respond more to higher doses, up to a maximum of 375 mg/day, generally in three divided doses (see PRECAUTIONS, General, Use in Patients with Concomitant Illness).
Special Populations
Treatment of Pregnant Women During the Third Trimester
Neonates exposed to venlafaxine hydrochloride tablets, other SNRIs, or SSRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (see PRECAUTIONS). When treating pregnant women with venlafaxine hydrochloride tablets during the third trimester, the physician should carefully consider the potential risks and benefits of treatment. The physician may consider tapering venlafaxine hydrochloride tablets in the third trimester.
Dosage for Patients with Hepatic Impairment
Given the decrease in clearance and increase in elimination half-life for both venlafaxine and ODV that is observed in patients with hepatic cirrhosis and mild and moderate hepatic impairment compared to normal subjects (see CLINICAL PHARMACOLOGY), it is recommended that the total daily dose be reduced by 50% in patients with mild to moderate hepatic impairment. Since there was much individual variability in clearance between subjects with cirrhosis, it may be necessary to reduce the dose even more than 50%, and individualization of dosing may be desirable in some patients.
Dosage for Patients with Renal Impairment
Given the decrease in clearance for venlafaxine and the increase in elimination half-life for both venlafaxine and ODV that is observed in patients with renal impairment (GFR = 10 to 70 mL/min) compared to normals (see CLINICAL PHARMACOLOGY), it is recommended that the total daily dose be reduced by 25% in patients with mild to moderate renal impairment. It is recommended that the total daily dose be reduced by 50% in patients undergoing hemodialysis. Since there was much individual variability in clearance between patients with renal impairment, individualization of dosing may be desirable in some patients.
Dosage for Elderly Patients
No dose adjustment is recommended for elderly patients on the basis of age. As with any antidepressant, however, caution should be exercised in treating the elderly. When individualizing the dosage, extra care should be taken when increasing the dose.
Maintenance Treatment
It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacological therapy beyond response to the acute episode. In one study, in which patients responding during 8 weeks of acute treatment with venlafaxine hydrochloride extended-release capsules were assigned randomly to placebo or to the same dose of venlafaxine hydrochloride extended-release capsules (75, 150, or 225 mg/day, qAM) during 26 weeks of maintenance treatment as they had received during the acute stabilization phase, longer-term efficacy was demonstrated. A second longer-term study has demonstrated the efficacy of venlafaxine hydrochloride tablets in maintaining an antidepressant response in patients with recurrent depression who had responded and continued to be improved during an initial 26 weeks of treatment and were then randomly assigned to placebo or venlafaxine hydrochloride tablets for periods of up to 52 weeks on the same dose (100 to 200 mg/day, on a b.i.d. schedule) (see CLINICAL TRIALS). Based on these limited data, it is not known whether or not the dose of venlafaxine hydrochloride tablets/venlafaxine hydrochloride extended-release capsules needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
Discontinuing Venlafaxine Hydrochloride Tablets
Symptoms associated with discontinuation of venlafaxine hydrochloride tablets, other SNRIs, and SSRIs, have been reported (see PRECAUTIONS). Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
SWITCHING PATIENTS TO OR FROM A MONOAMINE OXIDASE INHIBITOR
At least 14 days should elapse between discontinuation of an MAOI and initiation of therapy with venlafaxine hydrochloride tablets. In addition, at least 7 days should be allowed after stopping venlafaxine hydrochloride tablets before starting an MAOI (see CONTRAINDICATIONS ).
HOW SUPPLIED
Venlafaxine Hydrochloride Tablets equivalent to 25 mg of venlafaxine are peach-colored, round, flat, beveled-edged tablets with bisect on one side; one side of bisect is debossed with logo of "ZC" and other side is debossed with "64" and other side is plain and are supplied as follows:
NDC 68382-018-06 in bottles of 30 tablets
NDC 68382-018-14 in bottles of 60 tablets
NDC 68382-018-16 in bottles of 90 tablets
NDC 68382-018-01 in bottles of 100 tablets
NDC 68382-018-05 in bottles of 500 tablets
NDC 68382-018-10 in bottles of 1000 tablets
Venlafaxine Hydrochloride Tablets equivalent to 37.5 mg of venlafaxine are peach-colored, round, flat, beveled-edged tablets with bisect on one side; one side of bisect is debossed with logo of "ZC" and other side is debossed with "65" and other side is plain and are supplied as follows:
NDC 68382-019-06 in bottles of 30 tablets
NDC 68382-019-14 in bottles of 60 tablets
NDC 68382-019-16 in bottles of 90 tablets
NDC 68382-019-01 in bottles of 100 tablets
NDC 68382-019-05 in bottles of 500 tablets
NDC 68382-019-10 in bottles of 1000 tablets
Venlafaxine Hydrochloride Tablets equivalent to 50 mg of venlafaxine are peach-colored, round, flat, beveled-edged tablets with bisect on one side; one side of the bisect is debossed with logo of "ZC" and other side is debossed with "66" and other side is plain and are supplied as follows:
NDC 68382-020-06 in bottles of 30 tablets
NDC 68382-020-14 in bottles of 60 tablets
NDC 68382-020-16 in bottles of 90 tablets
NDC 68382-020-01 in bottles of 100 tablets
NDC 68382-020-05 in bottles of 500 tablets
NDC 68382-020-10 in bottles of 1000 tablets
Venlafaxine Hydrochloride Tablets equivalent to 75 mg of venlafaxine are peach-colored, round, flat, beveled-edged tablets with bisect on one side; one side of the bisect is debossed with logo of "ZC" and other side is debossed with "67" and other side is plain and are supplied as follows:
NDC 68382-021-06 in bottles of 30 tablets
NDC 68382-021-14 in bottles of 60 tablets
NDC 68382-021-16 in bottles of 90 tablets
NDC 68382-021-01 in bottles of 100 tablets
NDC 68382-021-05 in bottles of 500 tablets
NDC 68382-021-10 in bottles of 1000 tablets
Venlafaxine Hydrochloride Tablets equivalent to 100 mg of venlafaxine are peach-colored, round, flat, beveled-edged tablets with bisect on one side; one side of the bisect is debossed with logo of "ZC" and other side is debossed with "68" and other side is plain and are supplied as follows:
NDC 68382-101-06 in bottles of 30 tablets
NDC 68382-101-14 in bottles of 60 tablets
NDC 68382-101-16 in bottles of 90 tablets
NDC 68382-101-01 in bottles of 100 tablets
NDC 68382-101-05 in bottles of 500 tablets
NDC 68382-101-10 in bottles of 1000 tablets
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] in a dry place.
Dispense in a well-closed container as defined in the USP.
Medication Guide
Venlafaxine Hydrochloride Tablets
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member’s antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.
Talk to your, or your family member’s, healthcare provider about:
all risks and benefits of treatment with antidepressant medicines
all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
| • thoughts about suicide or dying |
• trouble sleeping (insomnia) |
| • attempts to commit suicide |
• new or worse irritability |
| • new or worse depression |
• acting aggressive, being angry, or violent |
| • new or worse anxiety |
• acting on dangerous impulses |
| • feeling very agitated or restless |
• an extreme increase in activity and talking (mania) |
| • panic attacks |
• other unusual changes in behavior or mood |
What else do I need to know about antidepressant medicines?
Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
Please address medical inquiries to, (MedicalAffairs@zydususa.com) Tel.: 1-877-993-8779.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Manufactured by:
Cadila Healthcare Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals USA Inc.
Pennington NJ 08534
Rev.: 12/10
Revision Date: 16/12/2010
NDC 60760-121-30
VENLAFAXINE
HYDROCHLORIDE
Tablets
75mg
QTY: 30
LOT# XXXXXXX
EXP XX-XX
RX # 0000000
MANUFACTURED BY:
Cadila Healtchcare Ltd.
Ahmedabad, India
PACKAGED BY:
St. Mary's MPP
10860 MAVINEE DR.
ORO VALLEY, AZ 85737
MANAGED PHARMACY PROGRAMS
VENLAFAXINE
HYDROCHLORIDE
Tablets
75mg
QTY: 30
RX # 0000000
NDC 60760-121-30
LOT# XXXXXXX
EXP XX-XX
TAKE AS DIRECTED
Rx only STORE AT CONTROLLED ROOM TEMPERATURE 15° to 30°C (59° to 86°F)
Venlafaxine HydrochlorideVenlafaxine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||