UREA
Urea Cream 39%
FULL PRESCRIBING INFORMATION: CONTENTS*
- INDICATIONS:
- UREA CONTRAINDICATIONS:
- CLINICAL PHARMACOLOGY:
- PHARMACOKINETICS:
- WARNINGS AND PRECAUTIONS:
- UREA ADVERSE REACTIONS:
- UREA DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
FULL PRESCRIBING INFORMATION
Rx OnlyFOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
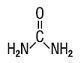
DESCRIPTION:
INDICATIONS:
CONTRAINDICATIONS:
CLINICAL PHARMACOLOGY:
PHARMACOKINETICS:
WARNINGS AND PRECAUTIONS:
FOR EXTERNAL USE ONLY.General:
Pregnancy: Pregnancy Category C.
Nursing Mothers:
ADVERSE REACTIONS:
You should call your doctor for medical advice about side effects. To report a serious adverse event, call 1-855-899-4237.DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:
KEEP OUT OF REACH OF CHILDREN.
Austin Pharmaceuticals, LLC

UREAUREA CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!