Urea
Urea Nail Stick 50% (in a vehicle containing lactic acid & zinc) Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
FOR EXTERNAL USE ONLY. AVOID CONTACT WITH EYES, LIPS OR MUCOUS MEMBRANES.
DESCRIPTION: Urea Nail Stick 50% is a keratolytic solution, which is gentle yet potent, tissue softener for nails. Each mL of Urea Nail Stick 50% contains 50% urea along with acrylates copolymer, carbomer, cetyl alcohol, disodium EDTA, dl-alphatocophery acetate, glycerin, lactic acid, linoleic acid, mineral oil, PEG-6, polysorbate 60, purified water, sodium hydroxide solution, stearic acid, titanium dioxide, zinc undecylenate.
Urea is a diamide of carbonic acid with the following chemical structure:
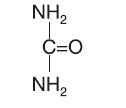
CLINICAL PHARMACOLOGY: Urea gently dissolves the intracellular matrix, which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual debridement of the nail plate.
PHARMACOKINETICS: The mechanism of action of topically applied urea is not yet known.
Uses
INDICATIONS AND USES: For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, devitalized, and ingrown nails.
CONTRAINDICATIONS: Known hypersensitivity to any of the listed ingredients.
WARNINGS: FOR EXTERNAL USE ONLY. Avoid contact with eyes, lips, inside the mouth/nose, and the vaginal/groin area. Consult your physician for directions about any areas of types of skin to apply or not apply the product. Tell your doctor if your condition persists or worsens.
PRECAUTIONS: This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY: Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however there are no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Urea Nail Stick 50% should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS: It is not known whether or not this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Urea Nail Stick 50% is administered to a nursing woman.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Call your doctor for medical advice about side effects.
DOSAGE AND ADMINISTRATION: Use this product as directed, and regularly, to achieve maximum benefit. Follow all directions on the product package and prescription label. If you are uncertain about any of the information, consult your doctor or pharmacist. Apply to the affected areas of the skin/nails, twice a day or as directed by your doctor. Rub in well until absorbed. As the product dries, the level of urea increases as the level of moisture decreases, allowing some of the urea to crystallize (white color). The product will work properly whether or not the urea can be seen on the surface. Wash hands after applying unless you are treating the hands.
HOW SUPPLIED: Urea Nail Stick 50% is supplied in a carton containing 6 nail sticks (2.4 mL each = 2.7 g each) NDC 42808-0205-15.
Store at controlled room temperature 15 to 30°C (59 to 86°F). Protect from freezing.
How to Dispense Urea Nail Stick 50% from the Container: Shake before use. Remove the cap, applicator side up. Holding the container with one hand, rotate the bottom dispensing dial counterclockwise. As the dial is rotated you will hear a clicking sound, which indicates that the product is being dispensed on the applicator. Rotate until the gel is visibly and apply as needed. After use, place the cap firmly on the container and when not in use keep the product tightly capped.
Manufactured in the U.S.A. for Exact-Rx, Inc., Melville, NY 11747
00-0205-15-205-00
Iss:6/11
PRINCIPAL DISPLAY PANEL
For External Use Only
NDC 42808-0205-15 Rx Only
Urea
In a vehicle containing
lactic acid & zinc
50%
NAIL STICKS
Exact-Rx.
INCORPORATED
Contains 6 Nail Sticks
(2.4 mL each)
UreaUrea EMULSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||