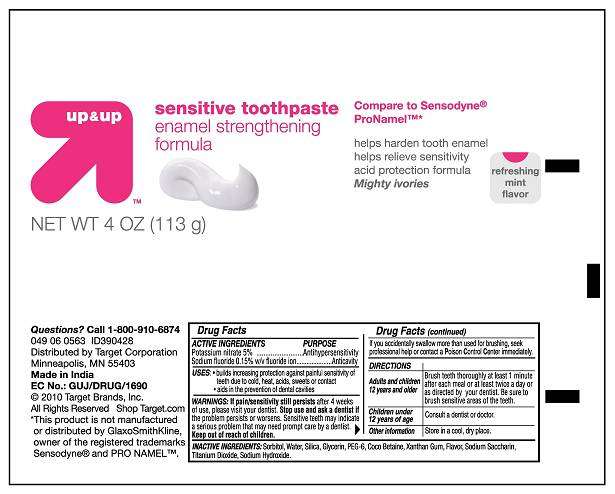
Up and UP Sensitive Toothpaste Enamel Strengthening formula
UP and Up Sensitive Toothpaste Enamel Strengthening formula
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredients
USES
- builds increasing protection against painful sensitivity of teeth due to cold, heat, acids, sweets or contact
- Aids in the prevention of dental cavities
WARNINGS
If pain/ sensitivity still persists after 4 weeks of use, please visit your dentist. Stop use and ask a dentist if the problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist. Keep out of reach of children. If you accidentally swallow more than used for brushing, seek professional help or contact a Poison Control Center immediately.
Directions
Adults and children 12 years and olderChildren under 12 years of age
Other information
Inactive ingredients:
Principal Display Panel
Purpose
Antihypersensitivity, Anticavity
KEEP OUT OF REACH OF CHILDREN
Up and UP Sensitive Toothpaste Enamel Strengthening formulaPotassium Nitrate and Sodium Fluoride PASTE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!