UP AND UP nasal decongestant
Target Corporation Nasal Decongestant Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- UP AND UP nasal decongestant Uses
- Warnings
- Directions
- UP AND UP nasal decongestant Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Pseudoephedrine HCl 30 mg
Purpose
Nasal decongestant
UP AND UP nasal decongestant Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
do not use more than directed
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days or occur with fever
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
| adults and children 12 years and over |
|
| children ages 6 to 12 years |
|
| children under 6 years | do not use this product in children under 6 years of age |
Other information
- each tablet contains: calcium 20 mg
- store at 20°-25°C (68°-77°F)
Inactive ingredients
carnauba wax, dibasic calcium phosphate dihydrate, FD&C red no. 40 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, silicon dioxide, titanium dioxide
Questions?
Call 1-800-910-6874
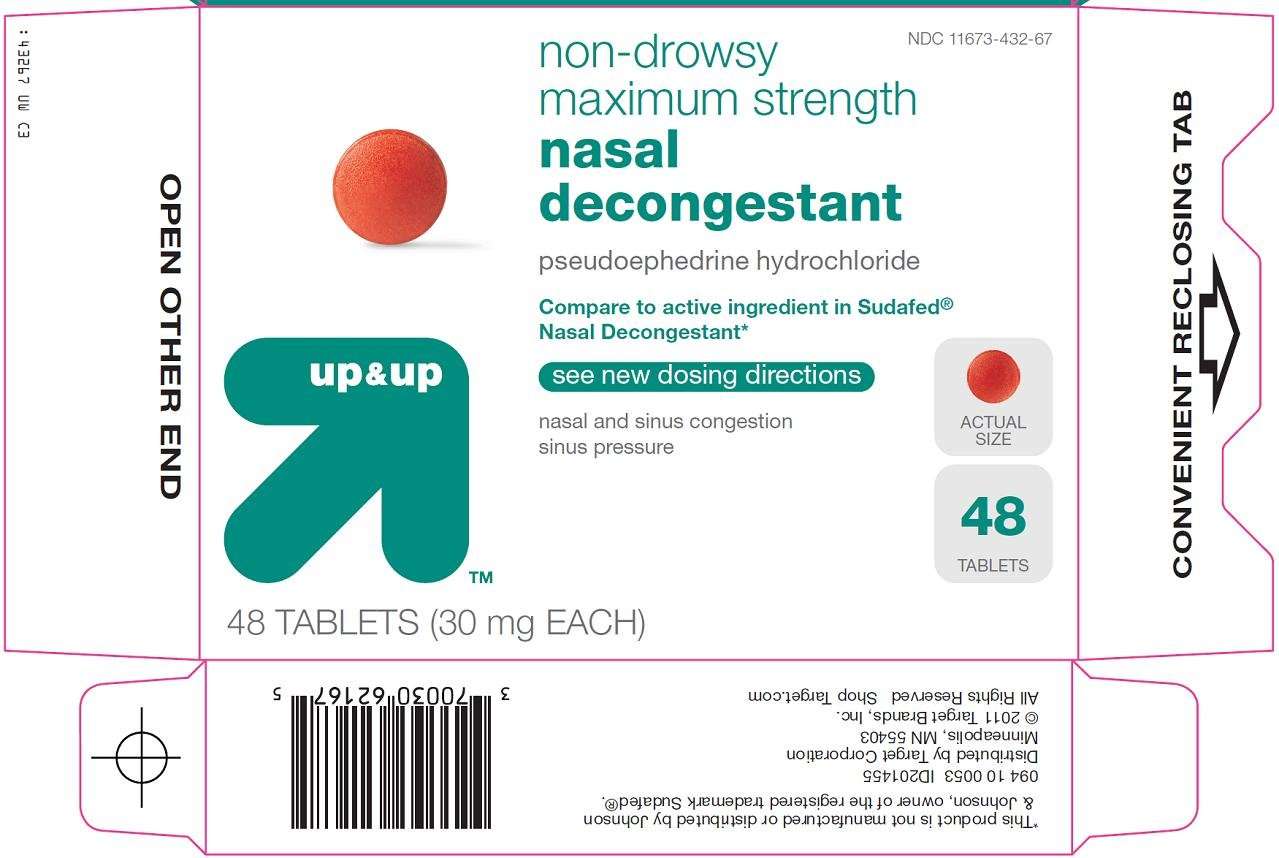
Principal Display Panel
non-drowsy
maximum strength
nasal decongestant
pseudoephedrine hydrochloride
Compare to active ingredient in Sudafed® Nasal Decongestant
see new dosing directions
nasal and sinus congestion
sinus pressure
ACTUAL SIZE
# TABLETS
(30 mg EACH)
UP AND UP nasal decongestantPseudoephedrine HCl TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||