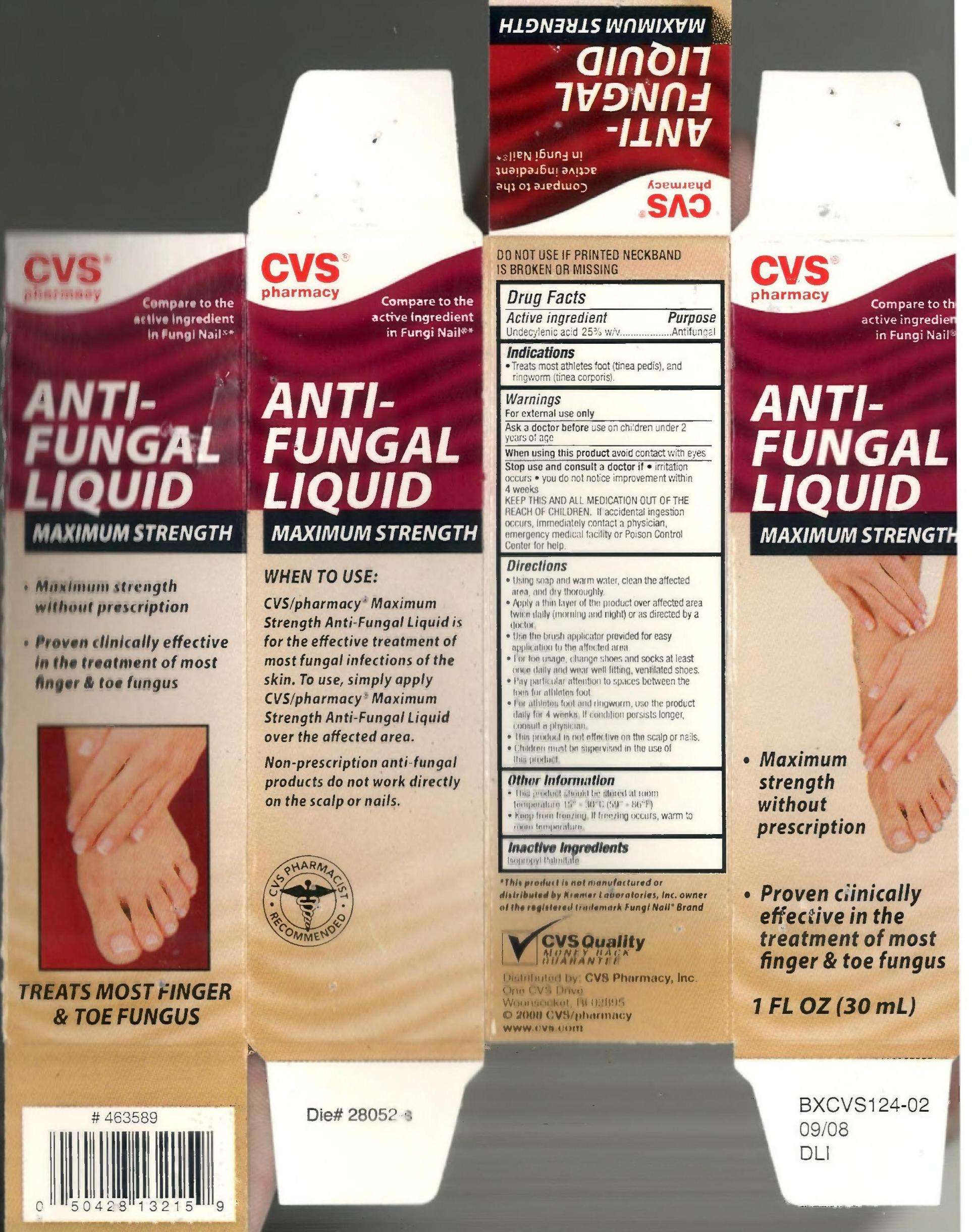
UNDECYLENIC ACID
drug facts
FULL PRESCRIBING INFORMATION
Active ingredient
Section from Carton Label Follows:Active ingredient Purpose
Purpose
Active ingredient Purpose
Uses
Warnings
KEEP THIS AND ALL MEDICATION OUT
OF THE REACH OF CHILDREN
Uses
Section from Directions portion of carton label follows:
Directions
- Using soap and water, clean the
affected area and dry thoroughly
- apply a thin layer of the product over
affected area twice daily (morning and
night) or as directed by a doctor.
- Use the brush applicator provided for
easy application to the affected area
- For toe usage, change shoes and socks
at least once daily and wear well fitting,
ventilated shoes
- Pay particular attention to spaces
between the toes for athletes foot
- For athletes foot and ringworm, use the
product daily for 4 weeks. If condition
persists longer, consult a physician
- This product is not effective on the scalp
or nails
- Children must be supervised in the use
of this product
Warnings For external use only.
______________________________
Ask a doctor before use
When using this product
Stop use and consult a doctor if
____________________________________
KEEP THIS AND ALL MEDICATION OUT
OF THE REACH OF CHILDREN
Directions
Inactive Ingredients
UNDECYLENIC ACIDUNDECYLENIC ACID LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||