Umcka FastActives Cherry
Umcka FastActives Cherry Packets
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient:
PELARGONIUM SIDOIDES
Inactive Ingredients:
Dosage and Administration:
Nature's Way Brand:
Directions:
Tear packet open, pour contents into mouth and
swallow. For best results, use at first sign of symptoms and continue to
use for an additional 48 hours after symptoms cease.
Adults and children 12 years of age and older: take contents of 1 packet 4-5
times daily.
Children 6-11 years of age: take contents of 1 packet 2-4 times daily.
Children under 6 years of age: consult a doctor.
Uses
Indications and Usage:
Shortens duration and reduces severity of symptoms associated with the common cold and throat/sinus/bronchial infections: congestion, cough, headache, hoarseness, minor aches, sore throat. Helps loosen phlegm (mucus) to make coughs more productive.
Purpose
Purpose:
Shortens duration and reduces severity of upper respiratory symptoms
Keep Out of Reach of Children:
Keep out of the reach of children.
Ask the Doctor:
Ask a doctor before use if you have a cough that lasts, is chronic such as occurs with smoking, asthma, or emphysema, or is accompanied by excessive phlegm (mucus).
Stop use and ask a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache. These could be signs of a serious condition.
Pregnancy or Breast Feeding:
If pregnant or breast-feeding, ask a healthcare professional before use.
Packaging Label. Principal Display Panel:
Umcka FastActives Cherry Packets 15347 Carton
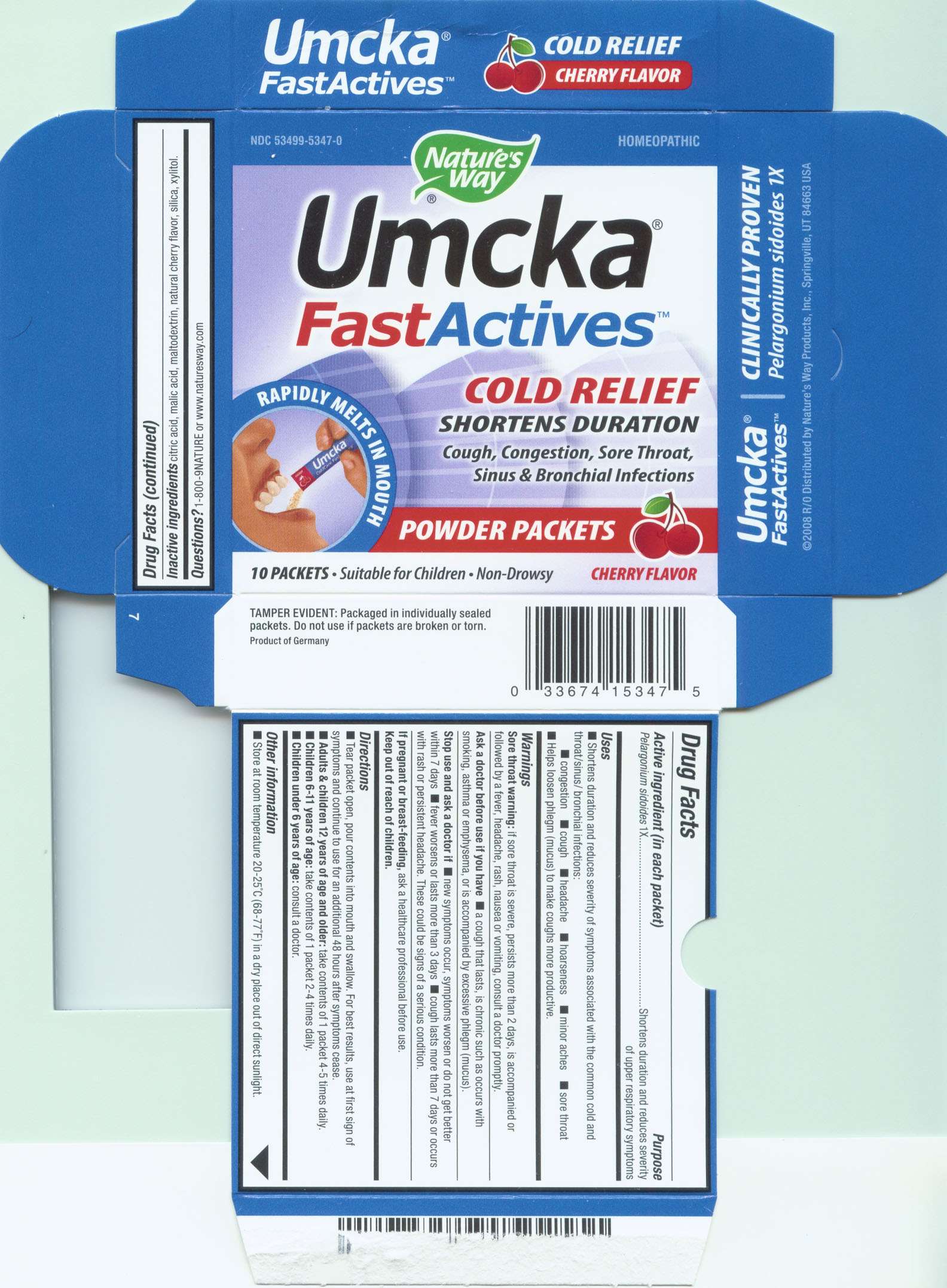
Umcka FastActives CherryPelargonium sidoides POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||