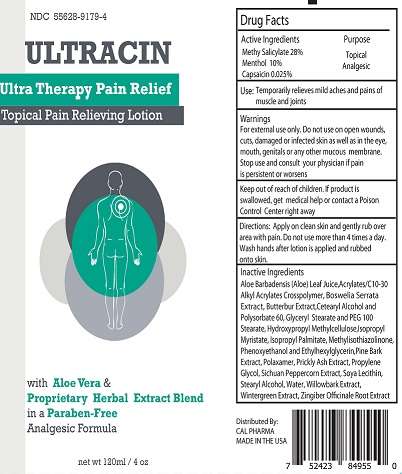
ULTRACIN
FULL PRESCRIBING INFORMATION
Active ingredient
METHYL SALICYLATE (28%)
MENTHOL (10%)
CAPSAICIN (0.025%)
Purpose
TOPICAL ANALGESIC
Uses
USE
- TEMPORARILY RELIEVES MILD ACHES AND PAINS OF MUSCLE AND JOINTS
WARNINGS
FOR EXTERNAL USE ONLY
DO NOT USE ON OPEN WOUNDS, CUTS, DAMAGED OR INFECTED SKIN AS WELL AS IN THE EYES, MOUTH, GENITALS OR OTHER MUCOUS MEMBRANE
STOP USE AND CONSULT YOUR PHYSICIAN IF PAIN IS PERSISTENT OR WORSENS
KEEP OUT OF REACH OF CHILDREN. IF PRODUCT IS SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- APPLY ON CLEAN SKIN AND GENTLY RUB OVER AREA WITH PAIN. DO NOT USE MORE THAN 4 TIMES A DAY. WASH HANDS AFTER LOTION IS APPLIED AND RUBBED ONTO SKIN.
INACTIVE INGREDIENTS
Aloe Barbadensis (Aloe) Leaf Juice,Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Boswelia Serrata Extract, Butterbur Extract,Cetearyl Alcohol and Polysorbate 60, Glyceryl Stearate and PEG 100 Stearate, Hydroxypropyl Methylcellulose,Isopropyl Myristate, Isopropyl Palmitate, Methylisothiazolinone, Phenoxyethanol and Ethylhexylglycerin,Pine Bark Extract, Polaxamer, Prickly Ash Extract, Propylene Glycol, Sichuan Peppercorn Extract, Soya Lecithin, Stearyl Alcohol, Water, Willowbark Extract, Wintergreen Extract, Zingiber Ocinale Root Extract
ULTRACINMETHYL SALICYLATE, MENTHOL, CAPSAICIN LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||