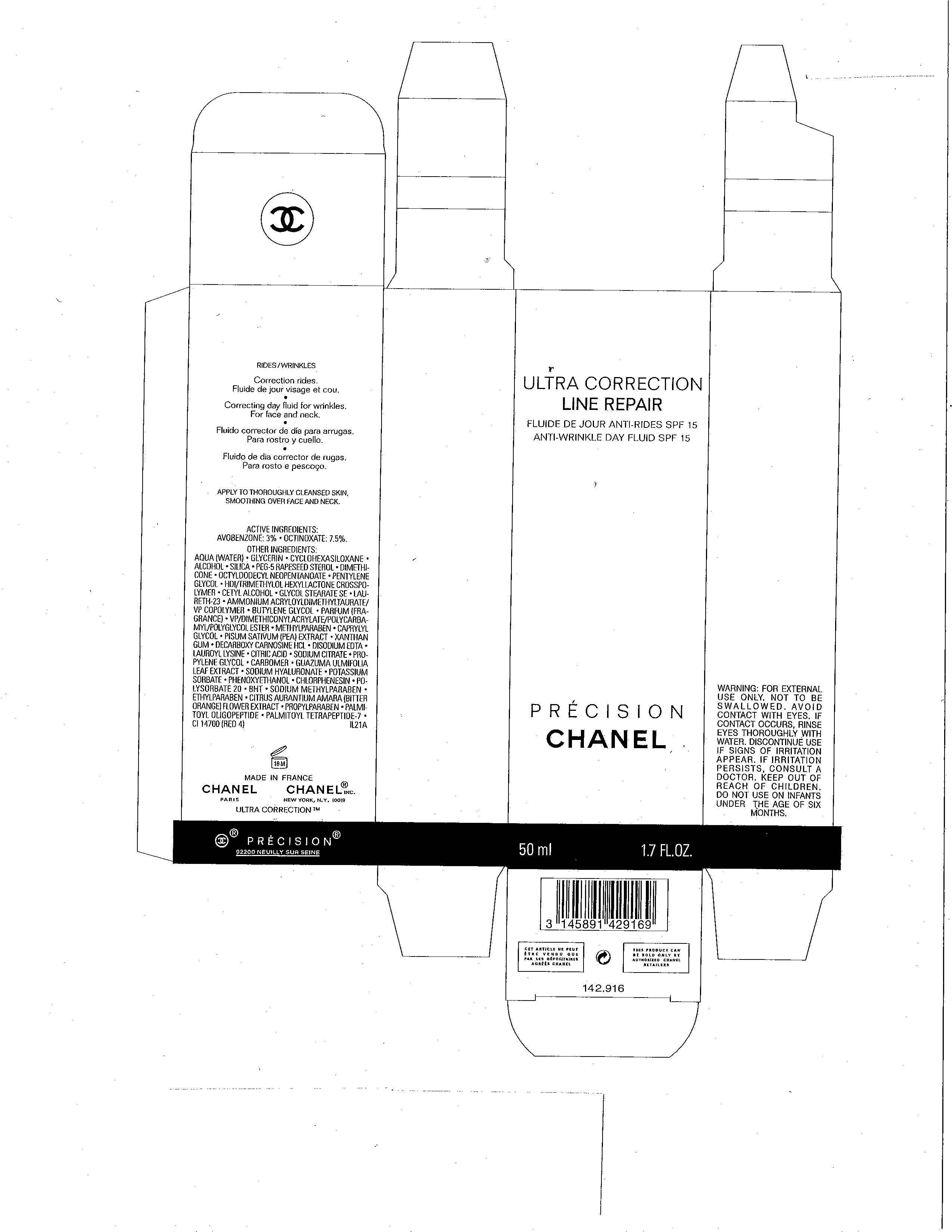
ULTRA CORRECTION
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
AVOBENZONE 3%
OCTINOXATE 7.5%
WARNINGS
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER. DISCONTINUE USE IF SIGNS OF IRRITATION PERSISTS. IF IRRITATION PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
APPLY TO THOROUGHLY CLEANSED SKIN, SMOOTHING OVER FACE AND NECK.
INACTIVE INGREDIENTS
AQUA (WATER), GLYCERIN, CYCLOHEXASILOXANE, ALCOHOL, SILICA, PEG-5 RAPESEED STEROL, DIMETHICONE, OCTYLDODECYL NEOPENTANOATE, PENTYLENE GLYCOL, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, CETYL ALCOHOL, GLYCOL STEARATE SE, LAURETH-23, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, BUTYLENE GLYCOL, PARFUM (FRAGRANCE), VP/DIMETHICONYLACRYLATE/POLYCARBAMYL/POLYGLYCOL ESTER, METHYLPARABEN, CAPRYLYL GLYCOL, PISUM SATIVUM (PEA) EXTRACT, XANTHAN GUM, DECARBOXY CARNOSINE HCL, DISODIUM EDTA, LAUROYL LYSINE, CITRIC ACID, SODIUM CITRATE, PROPYLENE GLYCOL, CARBOMER, GUAZUMA ULMIFOLIA LEAF EXTRACT, SODIUM HYALURONATE, POTASSIUM SORBATE, PHENOXYETHANOL, CHLORPHENESIN, POLYSORBATE 20, BHT, SODIUM METHYLPARABEN, ETHYLPARABEN, CITRUS AURANTIUM AMARA (BITTER ORANGE) FLOWER EXTRACT, PROPYLPARABEN, PALMITOYL OLIGOPEPTIDE, PALMITOYL TETRAPEPTIDE-7 CI 14700 (RED 4)
PRINCIPAL DISPLAY PANEL
ULTRA CORRECTION
LINE REPAIR
ANTI-WRINKLE DAY FLUID SPF 15
PRECISION
CHANEL
50 ml 1.7 FL.OZ
ULTRA CORRECTIONANTI-WRINKLE DAY FLUID EMULSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||