Tussin DM
Tussin DM
FULL PRESCRIBING INFORMATION: CONTENTS*
- Tussin DM Uses
- Warnings
- Tussin DM Other information
- Inactive ingredients
- Directions
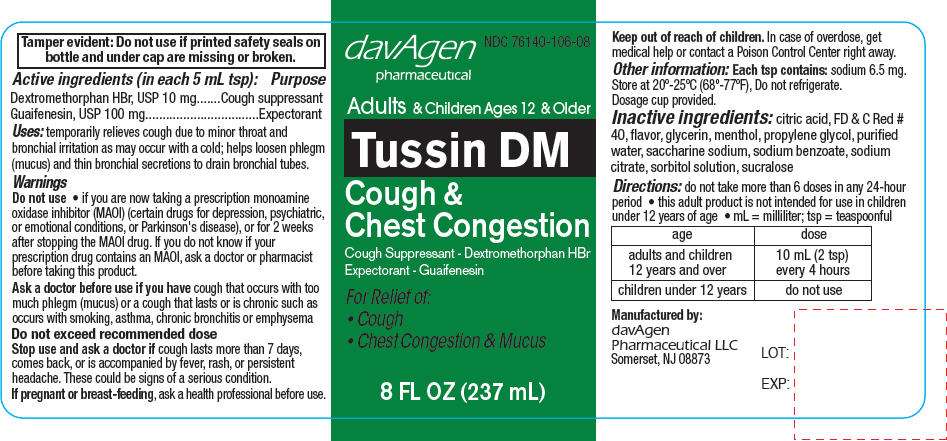
- PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
FULL PRESCRIBING INFORMATION
Active ingredient
Purpose
| Active ingredients (in each 5 mL tsp): | Purpose |
|---|---|
| Dextromethorphan HBr, USP 10 mg | Cough suppressant |
| Guaifenesin, USP 100 mg | Expectorant |
Tussin DM Uses
temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold; helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes.
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have cough that occurs with too much phlegm (mucus) or a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Do not exceed recommended dose
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Tussin DM Other information
Each tsp contains: sodium 6.5 mg. Store at 20°-25°C (68°-77°F), Do not refrigerate.
Dosage cup provided.
Inactive ingredients
citric acid, FD & C Red # 40, flavor, glycerin, menthol, propylene glycol, purified water, saccharine sodium, sodium benzoate, sodium citrate, sorbitol solution, sucralose
Directions
do not take more than 6 doses in any 24-hour period
- this adult product is not intended for use in children under 12 years of age
- mL = milliliter; tsp = teaspoonful
| age | dose |
|---|---|
| adults and children 12 years and over | 10 mL (2 tsp) every 4 hours |
| children under 12 years | do not use |
Manufactured by:
davAgen
Pharmaceutical LLC
Somerset, NJ 08873
PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
davAgen
pharmaceutical
NDC 76140-106-08
Adults & Children Ages 12 & Older
Tussin DM
Cough &
Chest Congestion
Cough Suppressant - Dextromethorphan HBr
Expectorant - Guaifenesin
For Relief of:
- Cough
- Chest Congestion & Mucus
8 FL OZ (237 mL)
Tussin DMDextromethorphan Hydrobromide and Guaifenesin LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||