Triple Antibiotic Plus Pain Relief
Navarro Discount Pharmacies,LLC
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients (in each gram)
- Purpose
- Triple Antibiotic Plus Pain Relief Uses
- Warnings
- Directions
- Triple Antibiotic Plus Pain Relief Other information
- Inactive ingredient
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Active ingredients (in each gram)
Purpose
First aid antibiotic
First aid antibiotic
First aid antibiotic
External analgesic
Triple Antibiotic Plus Pain Relief Uses
- cuts
- scrapes
- burns
Warnings
For external use only
Do not use
- if you are allergic to any of the ingredients
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- you need to use longer than 1 week
- condition persists or gets worse
- condition persists for more than 1 week, or clear up and occur again within a few days
- a rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a physician
Triple Antibiotic Plus Pain Relief Other information
Inactive ingredient
white petrolatum
PRINCIPAL DISPLAY PANEL
Triple Antibiotic Ointment Plus Pain Relief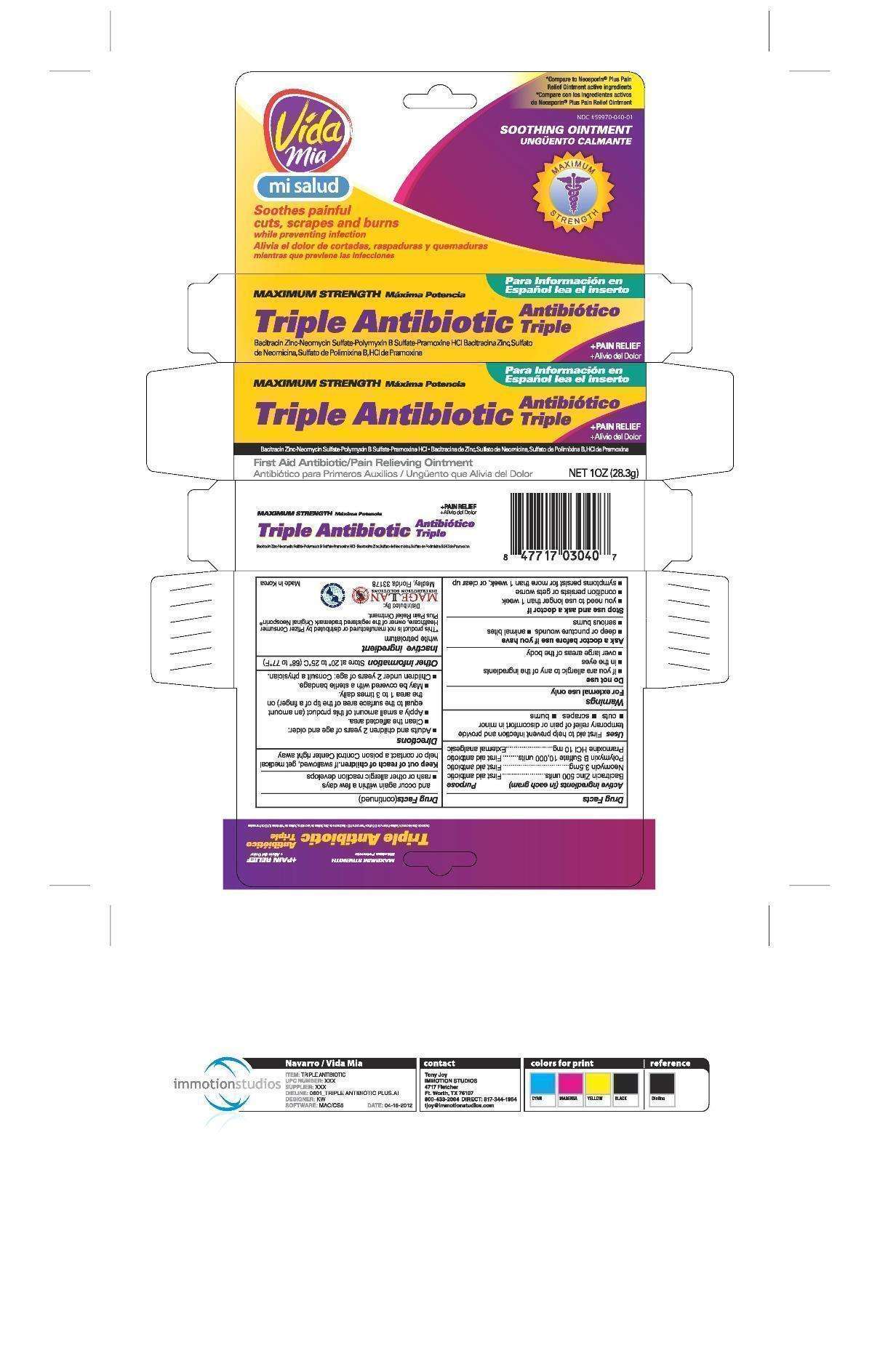
Triple Antibiotic Plus Pain ReliefBacitracin Zinc, Neomycin Sulfate, Polymyxin B Sulfate, and Pramoxine Hydrochloride OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!