Trimethobenzamide Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRIMETHOBENZAMIDE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- INDICATIONS & USAGE
- TRIMETHOBENZAMIDE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GERIATRIC USE
- TRIMETHOBENZAMIDE HYDROCHLORIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TRIMETHOBENZAMIDE HYDROCHLORIDE DESCRIPTION
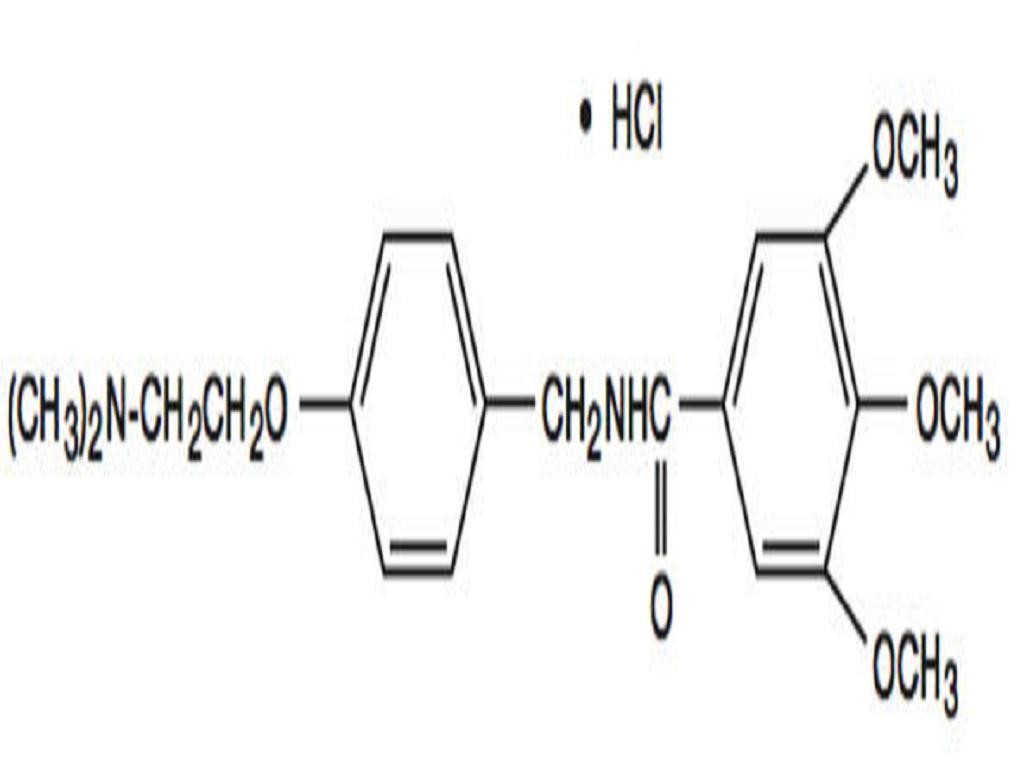
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS
USE IN SPECIFIC POPULATIONS
AgePRECAUTIONS: GeneralDOSAGE AND ADMINISTRATION
Gender
Race
Renal Impairment
PRECAUTIONS: GeneralDOSAGE AND ADMINISTRATION
INDICATIONS & USAGE
TRIMETHOBENZAMIDE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
General
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
TRIMETHOBENZAMIDE HYDROCHLORIDE ADVERSE REACTIONS
DOSAGE & ADMINISTRATION
WARNINGSPRECAUTIONSGeriatric Patients
CLINICAL PHARMACOLOGYPRECAUTIONS
Patients with Renal Impairment
CLINICAL PHARMACOLOGYPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Trimethobenzamide HydrochlorideTrimethobenzamide Hydrochloride CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!