Triamterene and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
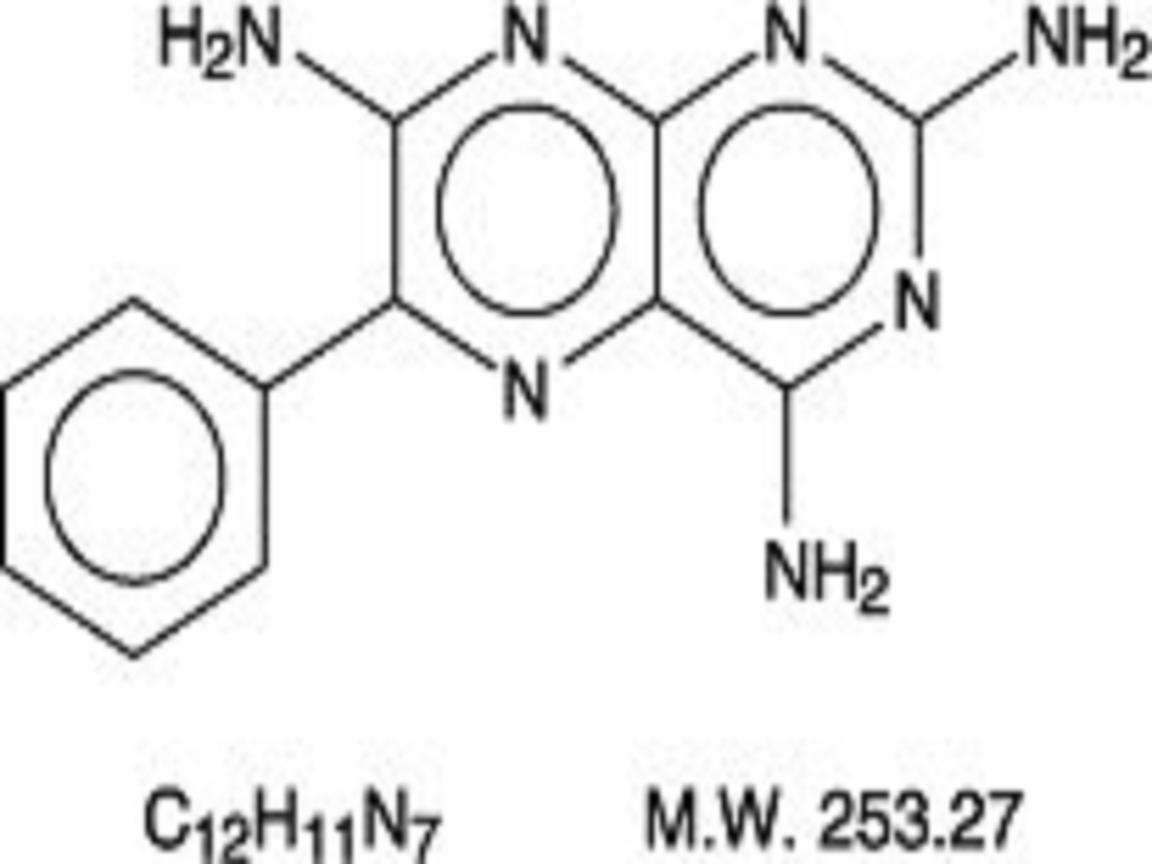
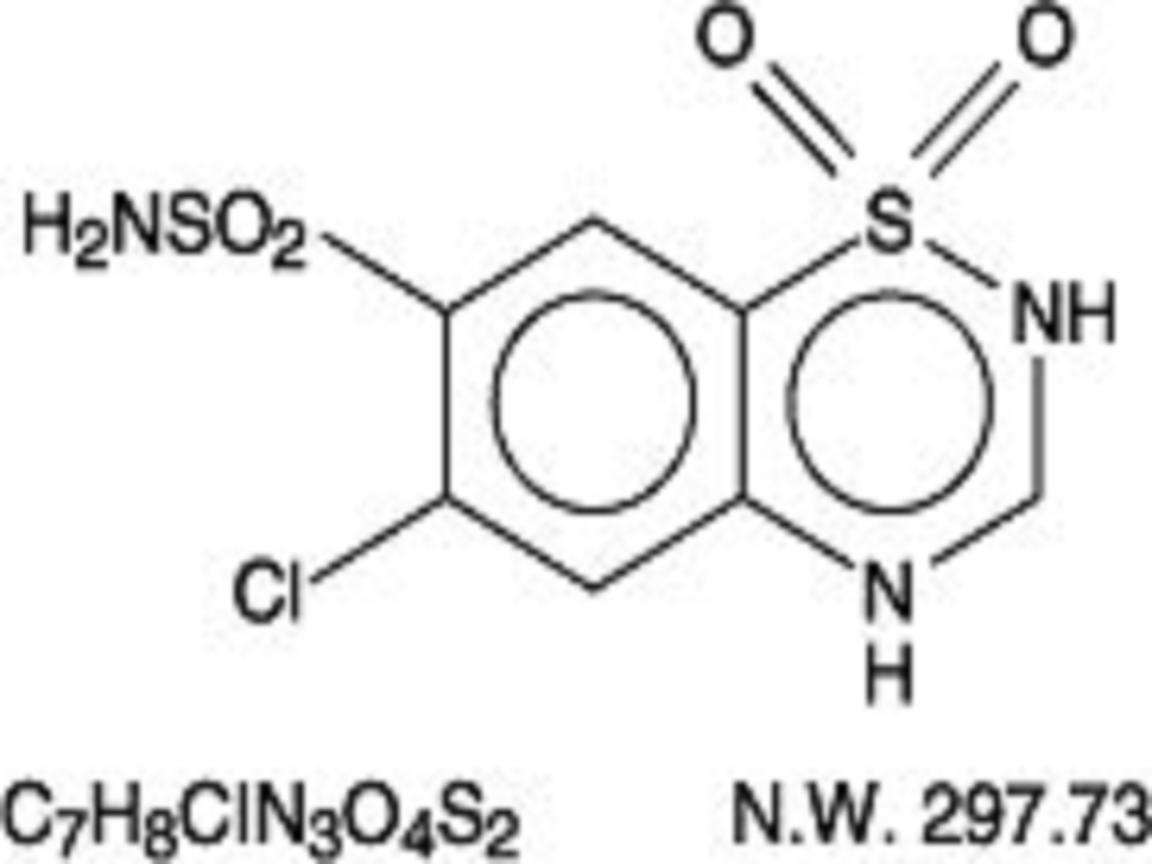
TRIAMTERENE AND HYDROCHLOROTHIAZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Triamterene
WARNINGS
Hydrochlorothiazide
INDICATIONS & USAGE
This fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked.Usage In Pregnancy
TRIAMTERENE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
HyperkalemiaAntikaliuretic Therapy or Potassium Supplementation
Impaired Renal Function
Hypersensitivity
WARNINGS
Hyperkalemia
CONTRAINDICATIONS
PRECAUTIONS: Drug Interactions
Metabolic or Respiratory Acidosis
Acute Myopia and Secondary Angle-Closure Glaucoma
PRECAUTIONS
General
ELECTROLYTE IMBALANCE AND BUN INCREASES
HEPATIC COMA
RENAL STONES
FOLIC ACID DEFICIENCY
HYPERURICEMIA
METABOLIC AND ENDOCRINE EFFECTS
HYPERSENSITIVITY
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisTriamterene
Hydrochlorothiazide
Mutagenesis
Triamterene
Hydrochlorothiazide
Impairment of Fertility
Hydrochlorothiazide
PREGNANCY
Category CTERATOGENIC EFFECTS
Triamterene and Hydrochlorothiazide
Triamterene
Hydrochlorothiazide
NONTERATOGENIC EFFECTS
NURSING MOTHERS
PEDIATRIC USE
TRIAMTERENE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Gastrointestinal:
Central Nervous System:
Cardiovascular:
Renal:
Hematologic:
Ophthalmic:
Hypersensitivity:
Other:
Altered Laboratory Findings
WARNINGSPRECAUTIONS
PRECAUTIONS
PRECAUTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGSWARNINGSPRECAUTIONS: Drug Interactions
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Triamterene and HydrochlorothiazideTriamterene and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!