TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
FULL PRESCRIBING INFORMATION: CONTENTS*
- HEPATOTOXICITY
- TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN INDICATIONS AND USAGE
- TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- Hepatoxicity
- Seizure Risk
- Suicide Risk
- Serotonin Syndrome Risk
- Hypersensitivity/Anaphylaxis
- Respiratory Depression
- Interaction With Central Nervous System (CNS) Depressants
- Interactions With Alcohol and Drugs of Abuse
- Increased Intracranial Pressure or Head Trauma
- Use in Ambulatory Patients
- Use With MAO Inhibitors and Serotonin Re-uptake Inhibitors
- Use With Alcohol
- Use With Other Acetaminophen-containing Products
- Misuse, Abuse and Diversion
- Risk of Overdosage
- Withdrawal
- PRECAUTIONS
- TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 37.5 mg/325 mg (100 Tablet Bottle)
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 37.5 mg/325 mg Blister Carton (8 x 10 Unit-dose)
FULL PRESCRIBING INFORMATION
HEPATOTOXICITY
Tramadol hydrochloride and acetaminophen tablets contains acetaminophen and tramadol HCl. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often involve more than one acetaminophen-containing product (see WARNINGS).
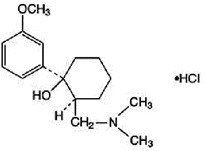
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN DESCRIPTION
cis
Np

®
®
CLINICAL PHARMACOLOGY
The following information is based on studies of tramadol alone or acetaminophen alone, except where otherwise noted:
Pharmacodynamics
CLINICAL PHARMACOLOGY, Pharmacokinetics
in vitro
Pharmacokinetics
|
Parametera
|
(+)- Tramadol
|
(-)- Tramadol
|
(+)- M1
|
(-)- M1
|
Acetaminophen
|
|||||
| Cmax(ng/mL) |
64.3 |
(9.3) |
55.5 |
(8.1) |
10.9 |
(5.7) |
12.8 |
(4.2) |
4.2 |
(0.8) |
| tmax(h) |
1.8 |
(0.6) |
1.8 |
(0.7) |
2.1 |
(0.7) |
2.2 |
(0.7) |
0.9 |
(0.7) |
| CL/F (mL/min) |
588 |
(226) |
736 |
(244) |
- |
- |
- |
- |
365 |
(84) |
| t1/2 (h) |
5.1 |
(1.4) |
4.7 |
(1.2) |
7.8 |
(3) |
6.2 |
(1.6) |
2.5 |
(0.6) |
max
NOO PRECAUTIONS, Drug Interactions
In vitro WARNINGS
Special Populations
DOSAGE AND ADMINISTRATION
PRECAUTIONS DOSAGE AND ADMINISTRATION
PRECAUTIONS, Geriatric Use
CLINICAL STUDIES
Single-Dose Studies for Treatment of Acute Pain
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN INDICATIONS AND USAGE
Tramadol hydrochloride and acetaminophen tablets USP are indicated for the short-term (five days or less) management of acute pain.
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN CONTRAINDICATIONS
Tramadol hydrochloride and acetaminophen tablets should not be administered to patients who have previously demonstrated hypersensitivity to tramadol, acetaminophen, any other component of this product, or opioids. Tramadol hydrochloride and acetaminophen tablets is contraindicated in any situation where opioids are contraindicated, including acute intoxication with any of the following: alcohol, hypnotics, narcotics, centrally acting analgesics, opioids, or psychotropic drugs. Tramadol hydrochloride and acetaminophen tablets may worsen central nervous system and respiratory depression in these patients.
WARNINGS
Hepatoxicity
Tramadol hydrochloride and acetaminophen tablets contains acetaminophen and tramadol HCl. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products (see Boxed Warning).
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4,000 milligrams of acetaminophen per day, even if they feel well.
Seizure Risk
Seizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous postmarketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. Concomitant use of tramadol increases the seizure risk in patients taking:
- Selective serotonin reuptake inhibitors (SSRI antidepressants or anorectics),
- Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or
- Other opioids.
Administration of tramadol may enhance the seizure risk in patients taking:
- MAO inhibitors (see also WARNINGS, Use with MAO Inhibitors and Serotonin Re-uptake Inhibitors),
- Neuroleptics, or
- Other drugs that reduce the seizure threshold.
Risk of convulsions may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, or CNS infections). In tramadol overdose, naloxone administration may increase the risk of seizure.
Suicide Risk
- Do not prescribe tramadol hydrochloride and acetaminophen tablets for patients who are suicidal or addiction-prone.
- Prescribe tramadol hydrochloride and acetaminophen tablets with caution for patients taking tranquilizers or antidepressant drugs and patients who use alcohol in excess and who suffer from emotional disturbance or depression.
WARNINGS, Risk of Overdosage
Serotonin Syndrome Risk
The development of a potentially life-threatening serotonin syndrome may occur with the use of tramadol products, including tramadol hydrochloride and acetaminophen tablets, particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs, and triptans, with drugs which impair metabolism of serotonin (including MAOIs), and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors). This may occur within the recommended dose (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Hypersensitivity/Anaphylaxis
CONTRAINDICATIONS
Respiratory Depression
Administer tramadol hydrochloride and acetaminophen tablets cautiously in patients at risk for respiratory depression. In these patients, alternative non-opioid analgesics should be considered. When large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose. If naloxone is to be administered, use cautiously because it may precipitate seizures (see WARNINGS, Seizure Risk and OVERDOSAGE ).
Interaction With Central Nervous System (CNS) Depressants
Tramadol hydrochloride and acetaminophen tablets should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers, or sedative hypnotics. Tramadol increases the risk of CNS and respiratory depression in these patients.
Interactions With Alcohol and Drugs of Abuse
Increased Intracranial Pressure or Head Trauma
Tramadol hydrochloride and acetaminophen tablets should be used with caution in patients with increased intracranial pressure or head injury. The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure and may be markedly exaggerated in these patients. Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reactions when evaluating altered mental status in these patients if they are receiving tramadol hydrochloride and acetaminophen tablets (see WARNINGS, Respiratory Depression ).
Use in Ambulatory Patients
Tramadol may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using this drug should be cautioned accordingly.
Use With MAO Inhibitors and Serotonin Re-uptake Inhibitors
Use tramadol hydrochloride and acetaminophen tablets with great caution in patients taking monoamine oxidase inhibitors. Animal studies have shown increased deaths with combined administration of MAO inhibitors and tramadol. Concomitant use of tramadol with MAO inhibitors or SSRI's increases the risk of adverse events, including seizure and serotonin syndrome.
Use With Alcohol
Tramadol hydrochloride and acetaminophen tablets should not be used concomitantly with alcohol consumption. The use of tramadol hydrochloride and acetaminophen tablets in patients with liver disease is not recommended.
Use With Other Acetaminophen-containing Products
Due to the potential for acetaminophen hepatotoxicity at doses higher than the recommended dose, tramadol hydrochloride and acetaminophen tablets should not be used concomitantly with other acetaminophen-containing products.
Misuse, Abuse and Diversion
DRUG ABUSE AND DEPENDENCE OVERDOSAGE
Risk of Overdosage
OVERDOSAGE
Withdrawal
Withdrawal symptoms may occur if tramadol hydrochloride and acetaminophen tablets is discontinued abruptly (see also DRUG ABUSE AND DEPENDENCE ). Reported symptoms have included: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Other symptoms that have been reported less frequently with tramadol hydrochloride and acetaminophen tablets discontinuation include: panic attacks, severe anxiety, and paresthesias. Clinical experience suggests that withdrawal symptoms may be avoided by tapering tramadol hydrochloride and acetaminophen tablets at the time of discontinuation.
PRECAUTIONS
General
WARNINGS, Use With Other Acetaminophen-containing Products Risk of Overdosage
Pediatric Use
The safety and effectiveness of tramadol hydrochloride and acetaminophen tablets has not been studied in the pediatric population.
Geriatric Use
In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function; of concomitant disease; and multiple drug therapy.
Acute Abdominal Conditions
The administration of tramadol hydrochloride and acetaminophen tablets may complicate the clinical assessment of patients with acute abdominal conditions.
Use in Renal Disease
Tramadol hydrochloride and acetaminophen tablets has not been studied in patients with impaired renal function. Experience with tramadol suggests that impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. In patients with creatinine clearances of less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride and acetaminophen tablets be increased, not to exceed 2 tablets every 12 hours.
Use in Hepatic Disease
Tramadol hydrochloride and acetaminophen tablets has not been studied in patients with impaired hepatic function. The use of tramadol hydrochloride and acetaminophen tablets in patients with hepatic impairment is not recommended (see WARNINGS, Use With Alcohol ).
Information for Patients
- Do not take tramadol hydrochloride and acetaminophen tablets if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing, stop taking tramadol hydrochloride and acetaminophen tablets and contact your healthcare provider immediately.
- Do not take more than 4,000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
- Do not take tramadol hydrochloride and acetaminophen tablets in combination with other tramadol or acetaminophen-containing products, including over-the-counter preparations.
- Tramadol hydrochloride and acetaminophen tablets may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SSRIs, SNRIs, and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
- Tramadol hydrochloride and acetaminophen tablets may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Tramadol hydrochloride and acetaminophen tablets should not be taken concomitantly with alcohol-containing beverages during the course of treatment with tramadol hydrochloride and acetaminophen tablets.
- Tramadol hydrochloride and acetaminophen tablets should be used with caution when taking medications such as tranquilizers, hypnotics, or other opiate-containing analgesics.
- Inform the physician if you are pregnant, think you might become pregnant, or are trying to become pregnant (see PRECAUTIONS, Labor and Delivery ).
- Understand the single-dose and 24-hour dose limit and the time interval between doses, since exceeding these recommendations can result in respiratory depression, seizures, hepatic toxicity, and death.
Drug Interactions
CLINICAL PHARMACOLOGY, Pharmacokinetics
WARNINGS, Serotonin Syndrome
WARNINGS, Serotonin Syndrome
carbamazepine
Quinidine In vitro
In vitro
In vitro
cimetidine
digoxin
Carcinogenesis, Mutagenesis, Impairment of Fertility
222
Salmonella
222
Pregnancy
Teratogenic Effects: Pregnancy Category C
22
Non-teratogenic Effects
22
Labor and Delivery
DRUG ABUSE AND DEPENDENCE
Nursing Mothers
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN ADVERSE REACTIONS
|
Body System
Preferred Term |
Tramadol hydrochloride and acetaminophen tablets
(N=142) (%) |
|
Gastrointestinal System Disorders
|
|
| Constipation |
6 |
| Diarrhea |
3 |
| Nausea |
3 |
| Dry Mouth |
2 |
|
Central & Peripheral Nervous System |
|
| Somnolence |
6 |
| Anorexia |
3 |
| Insomnia |
2 |
|
Central & Peripheral Nervous System |
|
| Dizziness |
3 |
|
Skin and Appendages |
|
| Sweating Increased |
4 |
| Pruritus |
2 |
|
Reproductive Disorders, Male
* |
|
| Prostatic Disorder |
2 |
*Number of males = 62
Incidence at least 1%, causal relationship at least possible or greater:
Body as a Whole
Central and Peripheral Nervous System
Gastrointestinal System
Psychiatric Disorders
Skin and Appendages
Selected Adverse events occurring at less than 1%
Body as a Whole
Cardiovascular Disorders
Central and Peripheral Nervous System
Gastrointestinal System
Hearing and Vestibular Disorders
Heart Rate and Rhythm Disorders
Liver and Biliary System
Metabolic and Nutritional Disorders
Psychiatric Disorders
Red Blood Cell Disorders
Respiratory System
Urinary System
Vision Disorders
Other clinically significant adverse experiences previously reported with tramadol hydrochloride.
Other clinically significant adverse experiences previously reported with acetaminophen.
DRUG ABUSE AND DEPENDENCE
Abuse
Tramadol has mu-opioid agonist activity, tramadol hydrochloride and acetaminophen tablets, a tramadol-containing product, can be abused and may be subject to criminal diversion.
Dependence
WARNINGS, Withdrawal
Generally, tolerance and/or withdrawal are more likely to occur the longer a patient is on continuous therapy with tramadol hydrochloride and acetaminophen tablets.
OVERDOSAGE
Tramadol
WARNINGS, Misuse, Abuse, and Diversion
Acetaminophen
acetaminophen
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN DOSAGE AND ADMINISTRATION
Individualization of Dose
HOW SUPPLIED
Micro Labs Limited
Micro Labs USA Inc.
Revised: 12/2012
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 37.5 mg/325 mg (100 Tablet Bottle)
NDC 42571-119-01
Tramadol
Hydrochloride and
Acetaminophen
Tablets, USP
37.5 mg/325 mg
Rx Only
100 Tablets
MICRO LABS

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 37.5 mg/325 mg Blister Carton (8 x 10 Unit-dose)
Tramadol Hydrochloride and
Acetaminophen Tablets, USP
37.5 mg/325 mg
Rx Only
80 (8x10) Unit-Dose Tablets
MICRO LABS
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHENTramadol hydrochloride and Acetaminophen TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||