Tramadal Hydrochloride and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PEDIATRIC USE
- GERIATRIC USE
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN DESCRIPTION
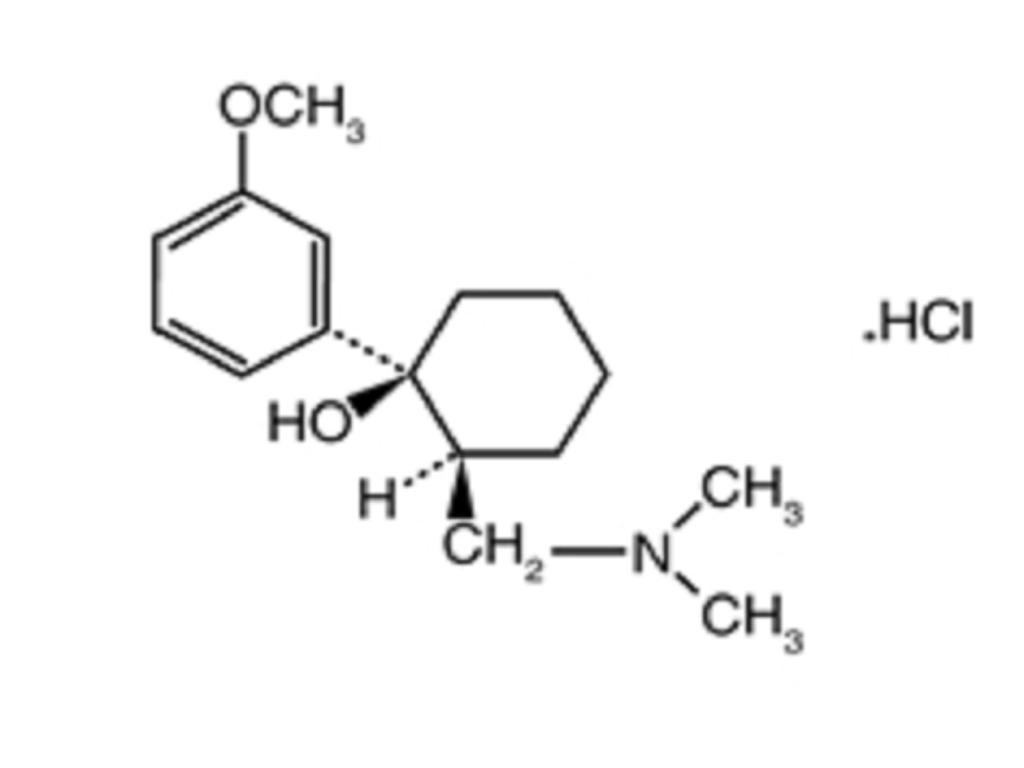
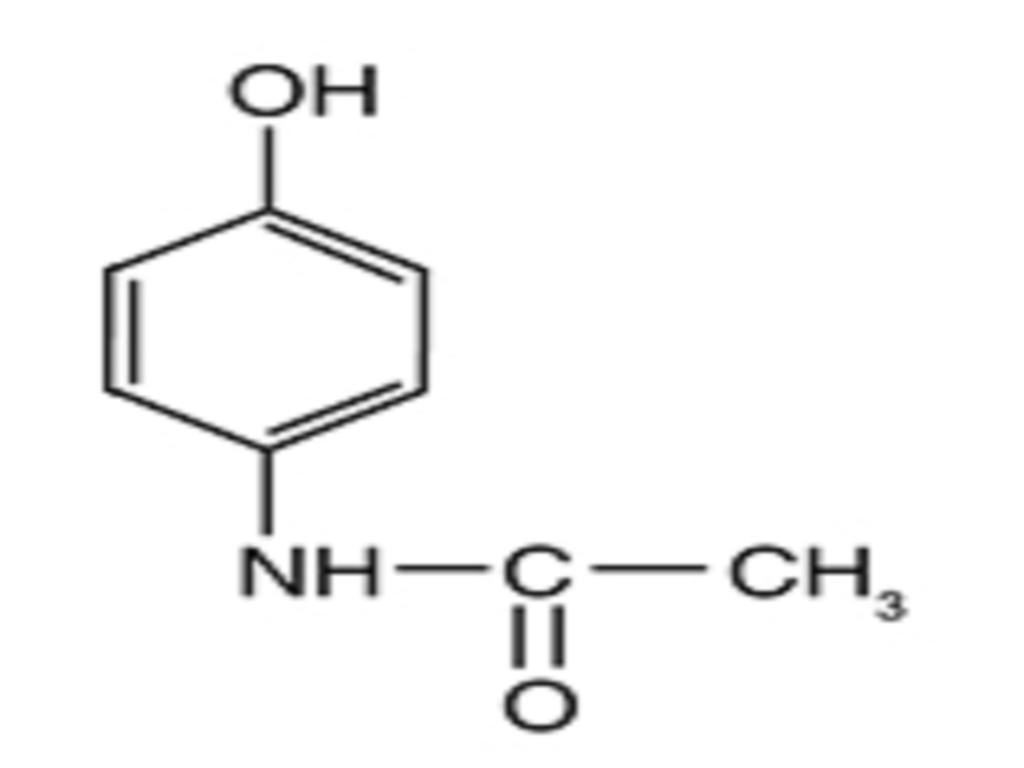
CLINICAL PHARMACOLOGY
The following information is based on studies of tramadol alone or acetaminophen alone, except where otherwise noted:PHARMACODYNAMICS
TramadolCLINICAL PHARMACOLOGY: Pharmacokinetics
Acetaminophen
PHARMACOKINETICS
**
Absorption
Food Effects
Distribution
Metabolism
PRECAUTIONS: Drug Interactions
WARNINGS
-
●
-
● conjugation with sulfate; and
-
● oxidation via the cytochrome, P450-dependent, mixed-function oxidase enzyme pathway to form a reactive intermediate metabolite, which conjugates with glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates. The principal cytochrome P450 isoenzyme involved appears to be CYP2E1, with CYP1A2 and CYP3A4 as additional pathways.
Elimination
USE IN SPECIFIC POPULATIONS
RenalDOSAGE AND ADMINISTRATION
Hepatic
PRECAUTIONSDOSAGE AND ADMINISTRATION
Geriatric
PRECAUTIONS: Geriatric Use
Gender
Pediatric
CLINICAL STUDIES
Single Dose Studies for Treatment of Acute PainINDICATIONS & USAGE
TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN CONTRAINDICATIONS
WARNINGS
Seizure Risk-
● Selective serotonin reuptake inhibitors (SSRI antidepressants or anorectics),
-
● Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or
-
● Other opioids.
-
● MAO inhibitors (see alsoWARNINGS: Use with MAO Inhibitors),
-
● Neuroleptics, or
-
● Other drugs that reduce the seizure threshold.
Anaphylactoid Reactions
CONTRAINDICATIONS
Respiratory Depression
WARNINGS: Seizure RiskOVERDOSAGE
Interaction With Central Nervous System (CNS) Depressants
Increased Intracranial Pressure or Head Trauma
Respiratory Depression
Use in Ambulatory Patients
Use With MAO Inhibitors and Serotonin Reuptake Inhibitors
Use With Alcohol
Use With Other Acetaminophen-containing Products
Withdrawal
DRUG ABUSE AND DEPENDENCE
Physical Dependence and Abuse
DRUG ABUSE AND DEPENDENCE
Risk of Overdosage
OVERDOSAGE
PRECAUTIONS
GeneralWARNINGS: Use With Other Acetaminophen-containing ProductsRisk of Overdosage
PEDIATRIC USE
GERIATRIC USE
Acute Abdominal Conditions
Use in Renal Disease
Use in Hepatic Disease
WARNINGS: Use With Alcohol
INFORMATION FOR PATIENTS
-
● Tramadol hydrochloride and acetaminophen tablets may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
-
● Tramadol hydrochloride and acetaminophen tablets should not be taken with alcohol-containing beverages.
-
● The patient should be instructed not to take tramadol hydrochloride and acetaminophen tablets in combination with other tramadol or acetaminophen-containing products, including over-the-counter preparations.
-
● Tramadol hydrochloride and acetaminophen tablets should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
-
● The patient should be instructed to inform the physician if they are pregnant, think they might become pregnant, or are trying to become pregnant (seePRECAUTIONS: Labor and Delivery).
-
● The patient should understand the single-dose and 24-hour dose limit and the time interval between doses, since exceeding these recommendations can result in respiratory depression, seizures, hepatic toxicity and death.
DRUG INTERACTIONS
Use With Carbamazepine
Use With Quinidine
Use With Inhibitors of CYP2D6
Use With Cimetidine
Use With MAO Inhibitors
WARNINGS: Use With MAO Inhibitors
Use With Digoxin
Use With Warfarin Like Compounds
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
LABOR & DELIVERY
DRUG ABUSE AND DEPENDENCENURSING MOTHERS
TRAMADAL HYDROCHLORIDE AND ACETAMINOPHEN ADVERSE REACTIONS
**
Incidence At Least 1%, Causal Relationship At Least Possible Or Greater
Body as a Whole:
Central and Peripheral Nervous System:
Gastrointestinal System:
Psychiatric Disorders:
Skin and Appendages:
Selected Adverse Events Occurring At Less Than 1%
Body as a Whole:
Cardiovascular Disorders
Central and Peripheral Nervous System:
Gastrointestinal System:
Hearing and Vestibular Disorders:
Heart Rate and Rhythm Disorders:
Liver and Biliary System:
Metabolic and Nutritional Disorders:
Psychiatric Disorders:
Red Blood Cell Disorders:
Respiratory System:
Urinary System:
Vision Disorders:
Other Clinically Significant Adverse Experiences Previously Reported With Tramadol Hydrochloride
Other Clinically Significant Adverse Experiences Previously Reported With Acetaminophen
DRUG ABUSE AND DEPENDENCE
WARNINGSOVERDOSAGE
Tramadol
WARNINGS
Acetaminophen
Treatment of Overdose
DOSAGE & ADMINISTRATION
Individualization of Dose
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Tramadal Hydrochloride and AcetaminophenTramadal Hydrochloride and Acetaminophen TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!