Tobramycin and Dexamethasone
H.J. Harkins Company, Inc.
Falcon Pharmaceuticals
FULL PRESCRIBING INFORMATION: CONTENTS*
- TOBRAMYCIN AND DEXAMETHASONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TOBRAMYCIN AND DEXAMETHASONE INDICATIONS AND USAGE
- TOBRAMYCIN AND DEXAMETHASONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- TOBRAMYCIN AND DEXAMETHASONE ADVERSE REACTIONS
- OVERDOSAGE
- TOBRAMYCIN AND DEXAMETHASONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESCRIPTION
Tobramycin and Dexamethasone Ophthalmic Suspension is a sterile, multiple dose antibiotic and steroid combination for topical ophthalmic use.
The chemical structures for tobramycin and dexamethasone are presented below:
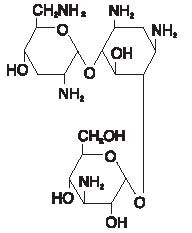

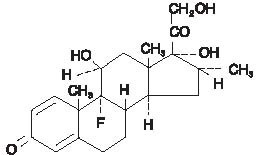

Each mL of Tobramycin and Dexamethasone Ophthalmic Suspension contains: Actives: tobramycin 0.3% (3 mg) and dexamethasone 0.1% (1 mg). Preservative: benzalkonium chloride 0.01%. Inactives: tyloxapol, edetate disodium, sodium chloride, hydroxyethyl cellulose, sodium sulfate, sulfuric acid and/or sodium hydroxide (to adjust pH) and purified water.
CLINICAL PHARMACOLOGY
Corticoids suppress the inflammatory response to a variety of agents and they probably delay or slow healing. Since corticoids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant. Dexamethasone is a potent corticoid.
The antibiotic component in the combination (tobramycin) is included to provide action against susceptible organisms. In vitro studies have demonstrated that tobramycin is active against susceptible strains of the following microorganisms:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
Bacterial susceptibility studies demonstrate that in some cases microorganisms resistant to gentamicin remain susceptible to tobramycin.
No data are available on the extent of systemic absorption from Tobramycin and Dexamethasone Ophthalmic Suspension; however, it is known that some systemic absorption can occur with ocularly applied drugs. If the maximum dose of Tobramycin and Dexamethasone Ophthalmic Suspension is given for the first 48 hours (two drops in each eye every 2 hours) and complete systemic absorption occurs, which is highly unlikely, the daily dose of dexamethasone would be 2.4 mg. The usual physiologic replacement dose is 0.75 mg daily. If Tobramycin and Dexamethasone Ophthalmic Suspension is given after the first 48 hours as two drops in each eye every 4 hours, the administered dose of dexamethasone would be 1.2 mg daily.
INDICATIONS AND USAGE
Tobramycin and Dexamethasone Ophthalmic Suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drug in this product is active against the following common bacterial eye pathogens:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
CONTRAINDICATIONS
Epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and many other viral diseases of the cornea and conjunctiva. Mycobacterial infection of the eye. Fungal diseases of ocular structures. Hypersensitivity to a component of the medication.
WARNINGS
NOT FOR INJECTION INTO THE EYE. Sensitivity to topically applied aminoglycosides may occur in some patients. If a sensitivity reaction does occur, discontinue use.
Prolonged use of steroids may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Intraocular pressure should be routinely monitored even though it may be difficult in pediatric patients and uncooperative patients. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. In acute purulent conditions of the eye, steroids may mask infection or enhance existing infection.
PRECAUTIONS
General
The possibility of fungal infections of the cornea should be considered after long-term steroid dosing. As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated. When multiple prescriptions are required, or whenever clinical judgement dictates, the patient should be examined with the aid of magnification, such as slit lamp biomicroscopy and, where appropriate, fluorescein staining.
Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the contents. Contact lenses should not be worn during the use of this product.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to evaluate the carcinogenic or mutagenic potential. No impairment of fertility was noted in studies of subcutaneous tobramycin in rats at doses of 50 and 100 mg/kg/day.
Pregnancy
Category C
Corticosteroids have been found to be teratogenic in animal studies. Ocular administration of 0.1% dexamethasone resulted in 15.6% and 32.3% incidence of fetal anomalies in two groups of pregnant rabbits. Fetal growth retardation and increased mortality rates have been observed in rats with chronic dexamethasone therapy. Reproduction studies have been performed in rats and rabbits with tobramycin at doses up to 100 mg/kg/day parenterally and have revealed no evidence of impaired fertility or harm to the fetus. There are no adequate and well controlled studies in pregnant women. Tobramycin and Dexamethasone Ophthalmic Suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tobramycin and Dexamethasone Ophthalmic Suspension is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
ADVERSE REACTIONS
Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination. Exact incidence figures are not available. The most frequent adverse reactions to topical ocular tobramycin [TOBREX®* (tobramycin ophthalmic solution)] are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than 4% of patients. Similar reactions may occur with the topical use of other aminoglycoside antibiotics. Other adverse reactions have not been reported; however, if topical ocular tobramycin is administered concomitantly with systemic aminoglycoside antibiotics, care should be taken to monitor the total serum concentration. The reactions due to the steroid component are: elevation of intraocular pressure (IOP) with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
Secondary Infection
The development of secondary infection has occurred after use of combinations containing steroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long term applications of steroids. The possibility of fungal invasion must be considered in any persistent corneal ulceration where steroid treatment has been used. Secondary bacterial ocular infection following suppression of host responses also occurs.
OVERDOSAGE
Clinically apparent signs and symptoms of an overdosage of Tobramycin and Dexamethasone Ophthalmic Suspension (punctate keratitis, erythema, increased lacrimation, edema and lid itching) may be similar to adverse reaction effects seen in some patients.
DOSAGE AND ADMINISTRATION
One or two drops instilled into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to one or two drops every two (2) hours. Frequency should be decreased gradually as warranted by improvement in clinical signs. Care should be taken not to discontinue therapy prematurely.
Not more than 20 mL should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above.
HOW SUPPLIED
Sterile ophthalmic suspension in 2.5 mL (NDC 61314-647-25), 5 mL (NDC 61314-647-05) and 10 mL (NDC 61314-647-10) DROP-TAINER®* dispensers.
STORAGE
Store at 8° to 27°C (46° to 80°F).
Store suspension upright and shake well before using.
Rx Only
*TOBREX and DROP-TAINER are registered trademarks of Alcon Research, Ltd.
340032-0608
Distributed by:
FALCON Pharmaceutical, Ltd.
Fort Worth, Texas 76134 USA
Manufactured by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
Revised: June 2008
Repacked by:
H.J. Harkins Company, Inc.
Nipomo, CA 93444 USA
PRINCIPAL DISPLAY PANEL
NDC 61314-647-05 Rx Only
FALCON
PHARMACEUTICALS®
Tobramycin and
Dexamethasone
Ophthalmic
Suspension
FOR TOPICAL OPHTHALMIC
USE ONLY
5 mL STERILE
AFFILIATE OF QUALITY RX
ALCON
LABORATORIES, INC.

Tobramycin and DexamethasoneTobramycin and Dexamethasone SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||