Thiothixene
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- THIOTHIXENE DESCRIPTION
- INDICATIONS & USAGE
- THIOTHIXENE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- THIOTHIXENE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- OVERDOSAGE
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGIncreased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thiothixene is not approved for the treatment of patients with dementia-related psychosis (seeWARNINGS).
THIOTHIXENE DESCRIPTION
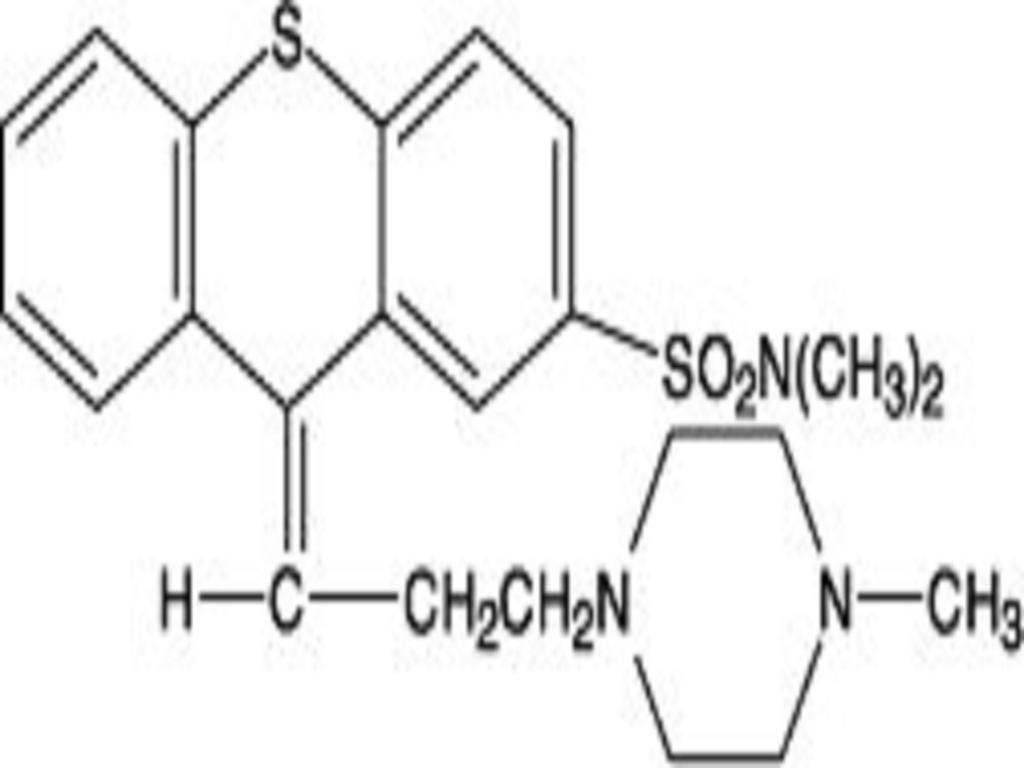
ACTIONS
INDICATIONS & USAGE
THIOTHIXENE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisElderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Thiothixene is not approved for the treatment of patients with dementia-related psychosis (seeBOXED WARNING).
Tardive Dyskinesia
Information for PatientsADVERSE REACTIONS
Neuroleptic Malignant Syndrome (NMS)
Usage in Pregnancy
Non-teratogenic Effects
Usage in Children
PRECAUTIONS
Leukopenia, Neutropenia and Agranulocytosis:
Class Effect
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
THIOTHIXENE ADVERSE REACTIONS
Cardiovascular Effects
CNS Effects
Extrapyramidal Symptoms
Dystonia: Class Effect
Dystonia
Class Effect
Persistent Tardive Dyskinesia
WARNINGS
Hepatic Effects
Hematologic Effects
Allergic Reactions
Endocrine/Reproductive
Autonomic Effects
Other Adverse Reactions
Neuroleptic Malignant Syndrome (NMS)
NMS
DOSAGE & ADMINISTRATION
OVERDOSAGE
Treatment
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ThiothixeneThiothixene CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!