TheradermSPF 43
Theraderm
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredients
Zinc Oxide 7.50% ...................Sunscreen
Octinoxate 7.50% ...................Sunscreen
Octisalate 2.50% ...................Sunscreen
TheradermSPF 43 Uses
Helps prevent sunburn and photo damage caused by UVA/UVB exposure.
Warnings
- For External use only.
- When using, avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Stop use and contact a doctor if irritation or rash develops.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Apply generously 15 minutes before sun exposure.
- Reapply to dry skin as needed.
- May be applied before make-up
Best used within 12 months of opening.
Inactive Ingredients
Purified water, Isopropyl palmitate, Octyl stearate, Ethyl hexyl isononanoate,Cyclopentasiloxane, Cetearyl glucoside, Dimethicone, Glycereth-26, Sodium Hyaluronate, Panthenol, Allantoin, Oleth-3 phosphate, Hydroxyethyl Acrylate/Sodium acryloyl dimethyl taurate copolymer, Polyisobutene, PEG-7 trimethylolpropane coconut ether, Polyether-1, Phenoxyethanol, Butylene glycol, Iodopropynyl butylcarbamate, Tocopheryl acetate, Triethoxycaprylylsilane.
Label for Tube and Box
Theraderm Tube Label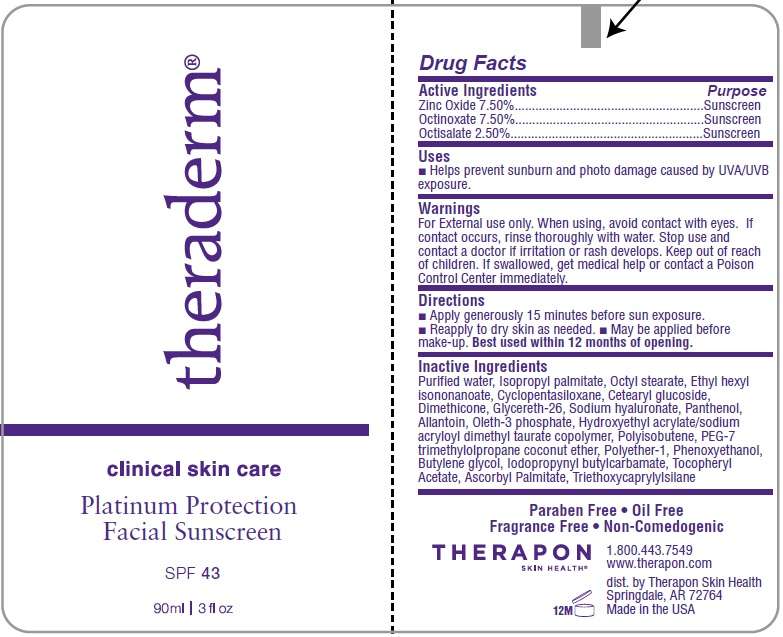
Theraderm Box Label
TheradermSPF 43Zinc Oxide, Octinoxate, Octisalate EMULSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||