TetraVisc
TetraVisc Hydrochloride Ophthalmic Solution, USP 0.5% Viscous Sterile Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- DESCRIPTON:
- CLINICAL PHARMACOLOGY:
- TETRAVISC INDICATIONS AND USAGE:
- General:
- Pregnancy Category C:
- Pediatric Use:
- TETRAVISC ADVERSE REACTIONS:
- TETRAVISC DOSAGE AND ADMINISTRATION: Dosage:
- HOW SUPPLIED:
- Storage:
FULL PRESCRIBING INFORMATION
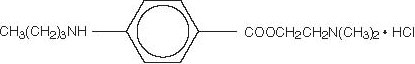
DESCRIPTON:
3(2)3 COOCH2CH2N(CH3)2.HCI
CLINICAL PHARMACOLOGY:
Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% acts by decreasing the permeability of the neuronal membrane,
thereby decreasing the flux of sodium, potassium and other ions associated with propagation of the nerve impulse. The onset
of anesthesia usually begins within 30 seconds and lasts a relatively short period of time.
INDICATIONS AND USAGE:
For procedures in which a rapid and short acting topical ophthalmic anesthetic is indicated such as in tonometry, gonioscopy,
removal of corneal foreign bodies, conjunctival scraping for diagnostic purposes, suture removal from the cornea or conjunctiva,
other short corneal and conjunctival procedures.
Should not be used by the patient without physician supervision, or in those persons showing hypersensitivity to any component
of this preparation.
For topical ophthalmic use only. Not for parenteral use. Not for injection. Do not use solution if it contains crystals or if it is cloudy
or discolored. Prolonged use results in diminished duration of anesthesia and retarded healing. This may cause the drug to be
used more frequently, creating a "vicious circle". Subsequent corneal infection and/or corneal opacification with accompanying
permanent visual loss or corneal perforation may occur. Prolonged use may also produce severe keratitis.
General:
Do not touch dropper tip to any surface as this may contaminate the solution. As with all anesthetics, continuous and prolonged
use should be avoided. Protection of the eye from irritating chemicals, foreign bodies and rubbing during the period of
anesthesia is very important. Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% should be used cautiously in patients
with known allergy or cardiac disease. If signs of sensitivity develop during the treatment or irritation persists or increases,
patients should be advised to discontinue use and consult prescribing physician.
Keep this and all drugs out of the reach of children . In case of accidental ingestion, seek professional assistance or contact a
Poison Control Center immediately.
After instillation of this product, the surface of the eye is insensitive and can be scratched without your feeling it. Do not rub
eye. Do not instill this product repeatedly because severe eye damage may occur.
No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Pregnancy Category C:
Animal reproduction studies have not been performed with Tetracaine Hydrochloride. It is also not known whether Tetracaine
Hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction. Tetracaine
Hydrochloride Ophthalmic Solution, USP 0.5% should be given to pregnant women only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should
be excercised when Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in children have not been established.
ADVERSE REACTIONS:
Transient symptoms (signs) such as stinging, burning, and conjunctival redness may occur. A rare, severe, immediate type
allergic corneal reaction has been reported characterized by acute diffuse epithelial keratitis with filament formation and/or
sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
To report SUSPECTED ADVERSE REACTIONS, contact OCuSOFT, Inc. at (800) 233-5469 www.ocusoft.com.
DOSAGE AND ADMINISTRATION: Dosage:
One to two drops per eye.
For Tonometry And Other Procedures Of Short Duration:
Instill one or two drops just prior to evaluation.
For Minor Surgical Procedures Such As Foreign Body Or Suture Removal:
Instill one or two drops in the eye(s) every five to ten minutes maximum three doses.
For Prolonged Anesthesia As In Cataract Extraction:
Instill one or two drops in the eye(s) every five to ten minutes maximum five doses.
HOW SUPPLIED:
Tetracaine Hydrochloride Ophthalmic Solution , USP 0.5% is supplied in:
5 mL multi-drop plastic container NDC 54799-505-05
0.6 mL single unit dose plastic container NDC 54799-505-01
Storage:
Store at a room temperature, 15°-30°C (59°-86°F).
Keep container tightly closed.


TetraViscTetracaine Hydrochloride LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||