Tetracycline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TETRACYCLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
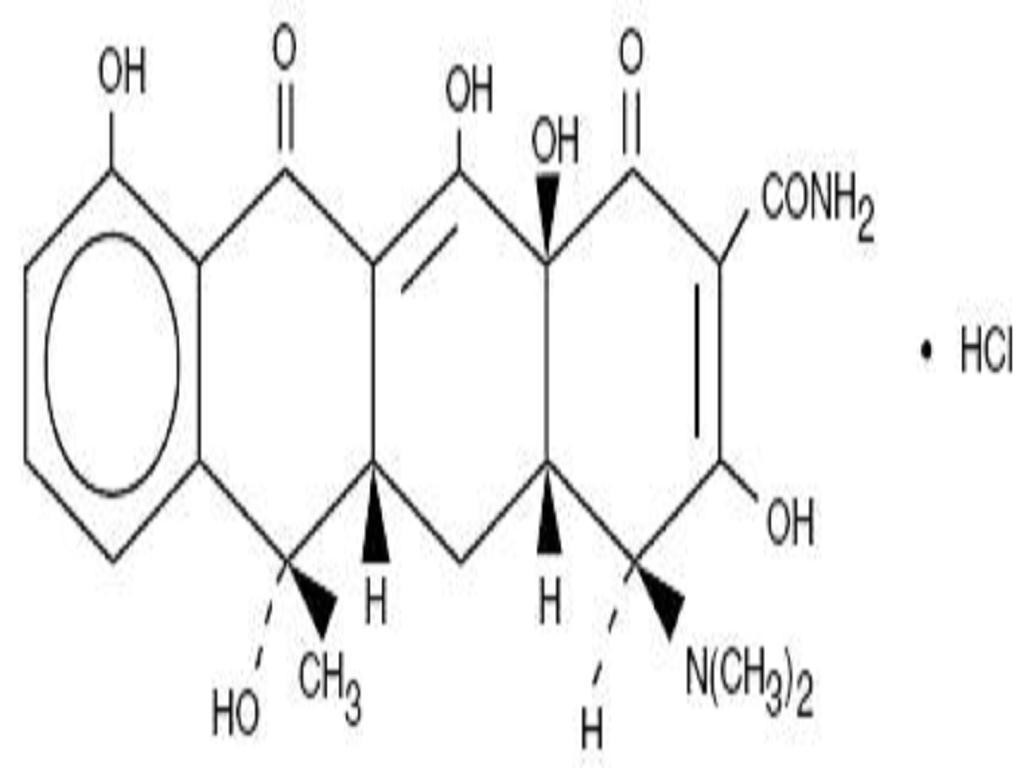
TETRACYCLINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Microbiology
Gram-negative Bacteria
Gram-positive Bacteria
Other microorganisms
Susceptibility Testing
INDICATIONS & USAGE
-
● Upper respiratory tract infections caused by Streptococcus pyogenes, Streptococcus pneumoniae and Hemophilus influenzae. Note: Tetracycline should not be used for streptococcal disease unless the organism has been demonstrated to be susceptible.
-
● Lower respiratory tract infections caused by Streptococcus pyogenes, Streptococcus pneumoniae, Mycoplasma pneumoniae (Eaton agent, and Klebsiella sp.)
-
● Skin and soft tissue infections caused by Streptococcus pyogenes, Staphylococcus aureaus. (Tetracyclines are not the drugs of choice in the treatment of any type of staphylococcal infections.)
-
● Infections caused by rickettsia including Rocky Mountain spotted fever, typhus group infections, Q fever, rickettsialpox.
-
● Psittacosis or ornithosis caused by Chlamydia Psittaci.
-
● Infections caused by Chlamydia trachomatis such as uncomplicated urethral, endocervical or rectal infections, inclusion conjunctivitis, trachoma, and lymphogranuloma venereum.
-
● Granuloma inquinale caused by Calymmatobacterium granulomatis.
-
● Relapsing fever caused by Borrelia sp.
-
● Bartonellosis caused by Bartonella bacilliformis.
-
● Chancroid caused by Hemophilus ducreyi.
-
● Tularemia caused by Francisella tularensis.
-
● Plaque caused by Yersinia pestis.
-
● Cholera caused by Vibrio cholerae.
-
● Brucellosis caused by Brucella species (tetracycline may be used in conjunction with an aminoglycoside).
-
● Infections due to Campylobacter fetus.
-
● As adjunctive therapy in intestinal amebiasis caused by Entamoeba histolytica.
-
● Urinary tract infections caused by susceptible strains of Escherichia coli, Klebsiella, etc.
-
● Other infections caused by susceptible gram-negative organisms such as E. coli, Enterobacter aerogenes, Shigella sp., Acinetobacter sp., Klebsiella sp., and Bacteroides sp.
-
● In severe acne, adjunctive therapy with tetracycline may be useful.
-
● When penicillin is contraindicated, tetracyclines are alternative drugs in the treatment of the following infections:
-
● syphilis and yaws caused by Treponema pallidum and pertenue, respectively,
-
● infections caused by Neisseria gonorrhoeae,
-
● anthrax caused by Bacillus anthracis,
-
● infections due to Listeria monocytogenes,
-
● actinomycosis caused by Actinomyces species,
-
● infections due to Clostridium species.
-
●
TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralInformation for Patients
Laboratory Tests
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic Effects
Pregnancy Category D
Nonteratogenic Effects
Labor and Delivery
Nursing Mothers
Pediatric Use
TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
AdultsChildren above eight years of age
Concomitant therapy
HOW SUPPLIED
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
INACTIVE INGREDIENT
lactosemagnesium stearate
sodium lauryl sulfate
DC yellow no. 10
FDC yellow no. 6
gelatin
titanium dioxide
benzyl alcohol
butylparaben
DC red no. 22
edetate calcium disodium
methylparaben
propylparaben
silicon dioxide
sodium propionate
pharmaceutical glaze
synthetic black iron oxide
DC yellow no. 10 aluminum lake
dimethylpolysiloxane
water
ethylene glycol monoethyl ether
FDC blue no. 1 aluminum lake
FDC blue no. 2 aluminum lake
FDC red no. 40 aluminum lake
lecithin
n-butyl alcohol
propylene alcohol
SDA-3A alcohol
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Tetracycline HydrochlorideTetracycline Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!