Tetracycline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- TETRACYCLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TETRACYCLINE HYDROCHLORIDE DESCRIPTION
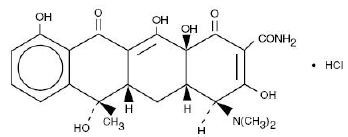
CLINICAL PHARMACOLOGY
Microbiology
INDICATIONS AND USAGEsection has not been documented.
Susceptibility Testing
INDICATIONS & USAGE
TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGS)
WARNINGS)
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
WARNINGSandDOSAGE AND ADMINISTRATION.TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
Gastrointestinal:anorexia, nausea, epigastric distress, vomiting, diarrhea, glossitis, black hairy tongue, dysphagia, enterocolitis, and inflammatory lesions (with monilial overgrowth) in the anogenital region.DOSAGE AND ADMINISTRATION).
Teeth:permanent discoloration of teeth may be caused during tooth development. Enamel hypoplasia has also been reported (seeWARNINGS).
Skin:maculopapular and erythematous rashes. Exfoliative dermatitis has been reported but is uncommon. Onycholysis and discoloration of the nails have been reported rarely. Photosensitivity is discussed inWARNINGS.
Renal toxicity:rise in BUN has been reported and is apparently dose related.
Liver:hepatotoxicity and liver failure have been observed in patients receiving large doses of tetracycline and in tetracycline-treated patients with renal impairment.
Hypersensitivity reactions:urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, pericarditis, exacerbation of systemic lupus erythematosus, and serum sickness-like reactions, as fever, rash, and arthralgia.
Blood:hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, neutropenia and eosinophilia have been reported.
Other:bulging fontanels in infants and intracranial pressure in adults (see PRECAUTIONS, General).
OVERDOSAGE
DOSAGE & ADMINISTRATION
AdultsChildren above eight years of age
WARNINGSand Carcinogenesis, Mutagenesis, Impairment of Fertility).
Concomitant therapy
WARNINGS): total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses.
ADVERSE REACTIONS).
HOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Tetracycline HydrochlorideTETRACYCLINE HYDROCHLORIDE CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!