Terazosin Hydrochloride
Bryant Ranch Prepack
Bryant Ranch Prepack
TERAZOSIN HYDROCHLORIDE CAPSULES
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERAZOSIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TERAZOSIN HYDROCHLORIDE INDICATIONS AND USAGE
- TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- TERAZOSIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- Terazosin Hcl 5mg Capsule
FULL PRESCRIBING INFORMATION
TERAZOSIN HYDROCHLORIDE DESCRIPTION
Terazosin hydrochloride, an alpha-1-selective adrenoceptor blocking agent, is a quinazoline derivative represented by the following structural formula, molecular formula and chemical name:
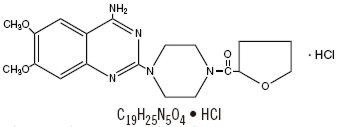
Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetra-hydro-2-furanyl)carbonyl]-, monohydrochloride, anhydrous.
Terazosin hydrochloride is a white, crystalline substance, freely soluble in water and isotonic saline and has a molecular weight of 459.93. Each terazosin hydrochloride capsule, for oral administration, contains 1 mg, 2 mg, 5 mg or 10 mg of terazosin as terazosin hydrochloride anhydrous. Each capsule contains the following inactive ingredients: crospovidone, lactose (monohydrate), magnesium stearate and microcrystalline cellulose. The capsule shells and imprinting inks contain: D & C Yellow # 10 Aluminum Lake, FD & C Blue # 1 Aluminum Lake, FD & C Blue # 2 Aluminum Lake, FD & C Red # 40 Aluminum Lake, gelatine, pharmaceutical glaze, propylene glycol, silicon dioxide, sodium lauryl sulfate, synthetic black iron oxide, and titanium dioxide. The 5 mg also contains; D & C Red # 28.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Pharmacokinetics
Terazosin hydrochloride administered as a capsule is essentially completely absorbed in man. Administration of capsules immediately after meals had a minimal effect on the extent of absorption. The time to reach peak plasma concentration however, was delayed by about 40 minutes. Terazosin has been shown to undergo minimal hepatic first-pass metabolism and nearly all of the circulating dose is in the form of parent drug. The plasma levels peak about one hour after dosing, and then decline with a half-life of approximately 12 hours. In a study that evaluated the effect of age on terazosin pharmacokinetics, the mean plasma half-lives were 14 and 11.4 hours for the age group ≥ 70 years and the age group of 20 to 39 years, respectively. After oral administration the plasma clearance was decreased by 31.7% in patients 70 years of age or older compared to that in patients 20 to 39 years of age.
The drug is 90 to 94% bound to plasma proteins and binding is constant over the clinically observed concentration range. Approximately 10% of an orally administered dose is excreted as parent drug in the urine and approximately 20% is excreted in the feces. The remainder is eliminated as metabolites. Impaired renal function had no significant effect on the elimination of terazosin, and dosage adjustment of terazosin to compensate for the drug removal during hemodialysis (approximately 10%) does not appear to be necessary. Overall, approximately 40% of the administered dose is excreted in the urine and approximately 60% in the feces. The disposition of the compound in animals is qualitatively similar to that in man.
TERAZOSIN HYDROCHLORIDE INDICATIONS AND USAGE
Terazosin hydrochloride is indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH). There is a rapid response, with approximately 70% of patients experiencing an increase in urinary flow and improvement in symptoms of BPH when treated with terazosin hydrochloride. The long-term effects of terazosin hydrochloride on the incidence of surgery, acute urinary obstruction or other complications of BPH are yet to be determined.
Terazosin hydrochloride is also indicated for the treatment of hypertension. It can be used alone or in combination with other antihypertensive agents such as diuretics or beta-adrenergic blocking agents.
TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
Terazosin hydrochloride capsules are contraindicated in patients known to be hypersensitive to terazosin hydrochloride.
WARNINGS
PRECAUTIONS
General
Information for Patients
(see ) Patient Package Insert
Patients should be made aware of the possibility of syncopal and orthostatic symptoms, especially at the initiation of therapy, and to avoid driving or hazardous tasks for 12 hours after the first dose, after a dosage increase and after interruption of therapy when treatment is resumed. They should be cautioned to avoid situations where injury could result should syncope occur during initiation of terazosin therapy. They should also be advised of the need to sit or lie down when symptoms of lowered blood pressure occur, although these symptoms are not always orthostatic, and to be careful when rising from a sitting or lying position. If dizziness, lightheadedness, or palpitations are bothersome they should be reported to the physician, so that dose adjustment can be considered.
Patients should also be told that drowsiness or somnolence can occur with terazosin, requiring caution in people who must drive or operate heavy machinery.
Patients should be advised about the possibility of priapism as a result of treatment with terazosin and other similar medications. Patients should know that this reaction to terazosin is extremely rare, but that if it is not brought to immediate medical attention, it can lead to permanent erectile dysfunction (impotence).
Laboratory Tests
Small but statistically significant decreases in hematocrit, hemoglobin, white blood cells, total protein and albumin were observed in controlled clinical trials. These laboratory findings suggested the possibility of hemodilution. Treatment with terazosin for up to 24 months had no significant effect on prostate specific antigen (PSA) levels.
Drug Interactions
In controlled trials, terazosin has been added to diuretics, and several beta-adrenergic blockers; no unexpected interactions were observed. Terazosin has also been used in patients on a variety of concomitant therapies; while these were not formal interaction studies, no interactions were observed. Terazosin has been used concomitantly in at least 50 patients on the following drugs or drug classes: 1) analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine, ibuprofen, indomethacin); 2) antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole); 3) anticholinergic/sympathomimetics (e.g., phenylephrine hydrochloride, phenylpropanolamine hydrochloride, pseudoephedrine hydrochloride); 4) antigout (e.g., allopurinol); 5) antihistamines (e.g., chlorpheniramine); 6) cardiovascular agents (e.g., atenolol, hydrochlorothiazide, methyclothiazide, propranolol); 7) corticosteroids; 8) gastrointestinal agents (e.g., antacids); 9) hypoglycemics; 10) sedatives and tranquilizers (e.g., diazepam).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Terazosin was devoid of mutagenic potential when evaluated and (the Ames test, cytogenetics, the dominant lethal test in mice, Chinese hamster chromosome aberration test and V79 forward mutation assay). in vivo in vitro in vivo in vivo
Terazosin, administered in the feed to rats at doses of 8, 40, and 250 mg/kg/day (70, 350, and 2100 mg/M /day), for two years, was associated with a statistically significant increase in benign adrenal medullary tumors of male rats exposed to the 250 mg/kg dose. This dose is 175 times the maximum recommended human dose of 20 mg (12 mg/M ). Female rats were unaffected. Terazosin was not oncogenic in mice when administered in feed for 2 years at a maximum tolerated dose of 32 mg/kg/day (110 mg/M ; 9 times the maximum recommended human dose). The absence of mutagenicity in a battery of tests, of tumorigenicity of any cell type in the mouse carcinogenicity assay, of increased total tumor incidence in either species, and of proliferative adrenal lesions in female rats, suggests a male rat species specific event. Numerous other diverse pharmaceutical and chemical compounds have also been associated with benign adrenal medullary tumors in male rats without supporting evidence for carcinogenicity in man. 2 2 2
The effect of terazosin on fertility was assessed in a standard fertility/reproductive performance study in which male and female rats were administered oral doses of 8, 30 and 120 mg/kg/day. Four of 20 male rats given 30 mg/kg (240 mg/M ; 20 times the maximum recommended human dose) and five of 19 male rats given 120 mg/kg (960 mg/M ; 80 times the maximum recommended human dose) failed to sire a litter. Testicular weights and morphology were unaffected by treatment. Vaginal smears at 30 and 120 mg/kg/day, however, appeared to contain less sperm than smears from control matings and good correlation was reported between sperm count and subsequent pregnancy. 2 2
Oral administration of terazosin for one or two years elicited a statistically significant increase in the incidence of testicular atrophy in rats exposed to 40 and 250 mg/kg/day (29 and 175 times the maximum recommended human dose), but not in rats exposed to 8 mg/kg/day (> 6 times the maximum recommended human dose). Testicular atrophy was also observed in dogs dosed with 300 mg/kg/day (> 500 times the maximum recommended human dose) for three months but not after one year when dosed with 20 mg/kg/day (38 times the maximum recommended human dose). This lesion has also been seen with prazosin, another selective alpha-1 blocking agent.
Pregnancy
Teratogenic Effects
Nonteratogenic Effects
In a peri- and post-natal development study in rats, significantly more pups died in the group dosed with 120 mg/kg/day (> 75 times the maximum recommended human dose) than in the control group during the 3 week postpartum period.
Nursing Mothers
It is not known whether terazosin is excreted in breast milk. Because many drugs are excreted in breast milk, caution should be exercised when terazosin is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been determined.
TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
Should overdosage of terazosin hydrochloride lead to hypotension, support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position. If this measure is inadequate, shock should first be treated with volume expanders. If necessary, vasopressors should then be used and renal function should be monitored and supported as needed. Laboratory data indicate that terazosin is 90 to 94% protein bound; therefore, dialysis may not be of benefit.
TERAZOSIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
If terazosin hydrochloride administration is discontinued for several days, therapy should be reinstituted using the initial dosing regimen.
When used to treat HYPERTENSION or BENIGN PROSTATIC HYPERPLASIA (BPH)
Please read this leaflet before you start taking terazosin hydrochloride capsules. Also, read it each time you get a new prescription. This is a summary and should NOT take the place of a full discussion with your doctor who has additional information about terazosin hydrochloride. You and your doctor should discuss terazosin hydrochloride and your condition before you start taking it and at your regular check-ups.
Terazosin hydrochloride capsules are used to treat high blood pressure (hypertension). Terazosin hydrochloride capsules are also used to treat benign prostatic hyperplasia (BPH) in men. This leaflet describes terazosin hydrochloride as a treatment for hypertension or BPH.
Blood pressure is the tension of the blood within the blood vessels. If blood is pumped too forcefully, or if the blood vessels are too narrow, the pressure of the blood against the walls of the vessels rises. What is Hypertension (High Blood Pressure)?
If high blood pressure is not treated, over time, the increased pressure can damage blood vessels or it can cause the heart to work too hard and may decrease the flow of blood to the heart, brain, and kidneys. As a result, these organs may become damaged and not function correctly. If high blood pressure is controlled, this damage is less likely to happen.
Nondrug treatments are sometimes effective in controlling mild hypertension. The most important lifestyle changes to lower blood pressure are to lose weight, reduce salt, fat, and alcohol in the diet, quit smoking, and exercise regularly. However, many hypertensive patients require one or more ongoing medications to control their blood pressure. There are different kinds of medications used to treat hypertension. Your doctor has prescribed terazosin hydrochloride for you. Treatment Options for Hypertension:
Terazosin hydrochloride works by relaxing blood vessels so that blood passes through them more easily. This helps to lower blood pressure. What Terazosin Hydrochloride Does to Treat Hypertension:
The prostate is a gland located below the bladder of men. It surrounds the urethra (you-REETH-rah), which is a tube that drains urine from the bladder. BPH is an enlargement of the prostate gland. The symptoms of BPH, however, can be caused by an increase in the tightness of muscles in the prostate. If the muscles inside the prostate tighten, they can squeeze the urethra and slow the flow of urine. This can lead to symptoms such as: What is BPH?
- a weak or interrupted stream when urinating
- a feeling that you cannot empty your bladder completely
- a feeling of delay when you start to urinate
- a need to urinate often, especially at night, or
- a feeling that you must urinate right away.
There are three main treatment options for BPH: Treatment Options for BPH:
- Program of monitoring or "Watchful Waiting". Some men have an enlarged prostate gland, but no symptoms, or symptoms that are not bothersome. If this applies, you and your doctor may decide on a program of monitoring including regular checkups, instead of medication or surgery.
- Medication. There are different kinds of medication used to treat BPH. Your doctor has prescribed terazosin hydrochloride for you. See "What Terazosin Hydrochloride Does to Treat BPH" below.
- Surgery. Some patients may need surgery. Your doctor can describe several different surgical procedures to treat BPH. Which procedure is best depends on your symptoms and medical condition.
Terazosin hydrochloride relaxes the tightness of a certain type of muscle in the prostate and at the opening of the bladder. This may increase the rate of urine flow and/or decrease the symptoms you are having. What Terazosin Hydrochloride Does to Treat BPH:
- Terazosin hydrochloride helps relieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
- If terazosin hydrochloride is helping you, you should notice an effect on your particular symptoms in 2 to 4 weeks of starting to take the medication.
- Even though you take terazosin hydrochloride and it may help you, terazosin hydrochloride may not prevent the need for surgery in the future.
Other Important Facts About Terazosin Hydrochloride for BPH:
- You should see an effect on your symptoms in 2 to 4 weeks. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular check-ups.
- Your doctor has prescribed terazosin hydrochloride for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking terazosin hydrochloride. Terazosin hydrochloride is not a treatment for prostate cancer.
- About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that terazosin hydrochloride does not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
You may feel dizzy, faint, or "lightheaded" particularly after you get up from bed or from a chair. This is more likely to occur after you've taken the first few doses, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then restart treatment. What You Should Know While Taking Terazosin Hydrochloride for Hypertension or BPH: Terazosin Hydrochloride Can Cause a Sudden Drop in Blood Pressure After the VERY FIRST DOSE. WARNINGS:
Because of this effect, your doctor may have told you to take terazosin hydrochloride at bedtime. If you take terazosin hydrochloride at bedtime but need to get up from bed to go to the bathroom, get up slowly and cautiously until you are sure how the medicine affects you. It is also important to get up slowly from a chair or bed at any time until you learn how you react to terazosin hydrochloride. You should not drive or do any hazardous tasks until you are used to the effects of the medication. If you begin to feel dizzy, sit or lie down until you feel better.
- You will start with a 1 mg dose of terazosin hydrochloride. Then the dose will be increased as your body gets used to the effect of the medication.
- Other side effects you could have while taking terazosin hydrochloride include drowsiness, blurred or hazy vision, nausea, or "puffiness" of the feet or hands. Discuss any unexpected effects you notice with your doctor.
Extremely rarely, terazosin hydrochloride and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to emergency room as soon as possible.
Follow your doctor's instructions about how to take terazosin hydrochloride capsules. You must take it every day at the dose prescribed. Talk with your doctor if you don't take it for a few days, you may have to restart it at a 1 mg dose and be cautious about possible dizziness. Do not share terazosin hydrochloride capsules with anyone else; it was prescribed only for you. How to Take Terazosin Hydrochloride Capsules:
Keep terazosin hydrochloride capsules and all medicines out of the reach of children.
Store at 15° to 30°C (59° to 86°F).
Protect from light and moisture.
FOR MORE INFORMATION ABOUT TERAZOSIN HYDROCHLORIDE CAPSULES AND HYPERTENSION OR BPH, TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Terazosin Hcl 5mg Capsule

Terazosin HydrochlorideTerazosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||