Terazosin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERAZOSIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TERAZOSIN HYDROCHLORIDE DESCRIPTION
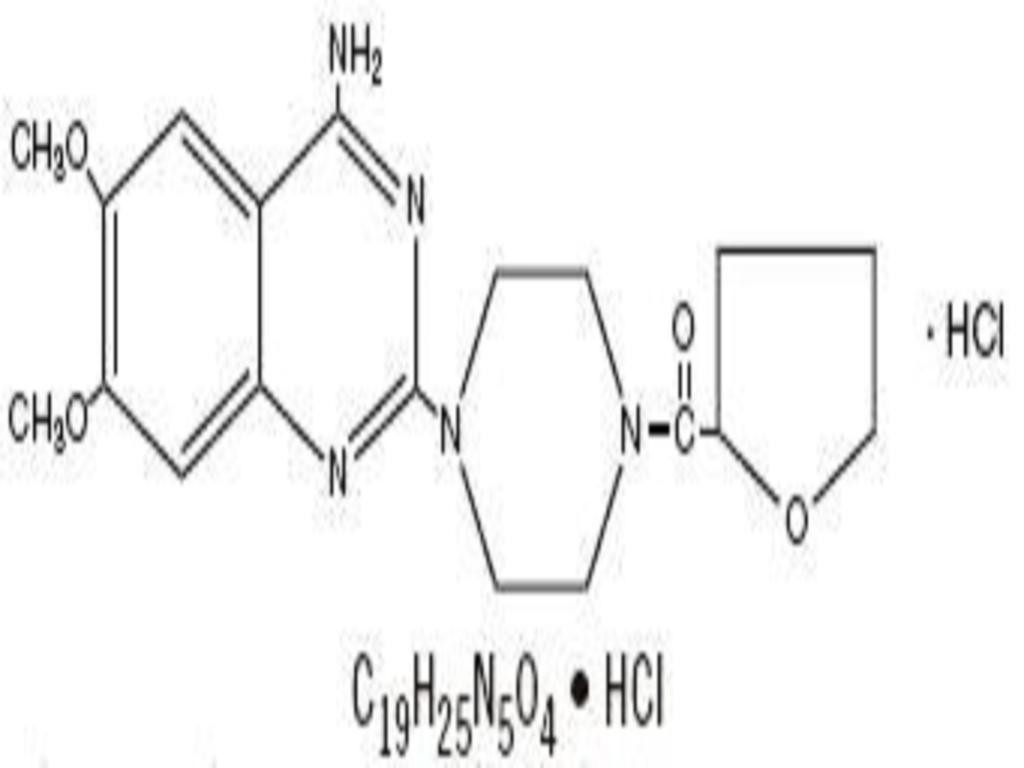
CLINICAL PHARMACOLOGY
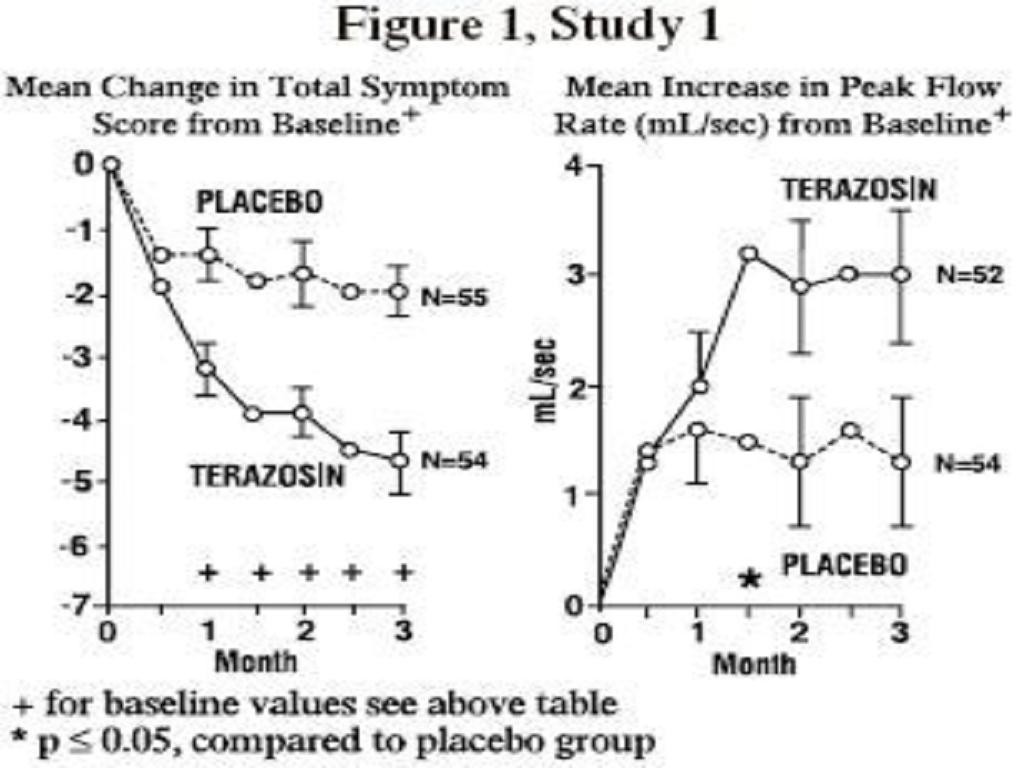
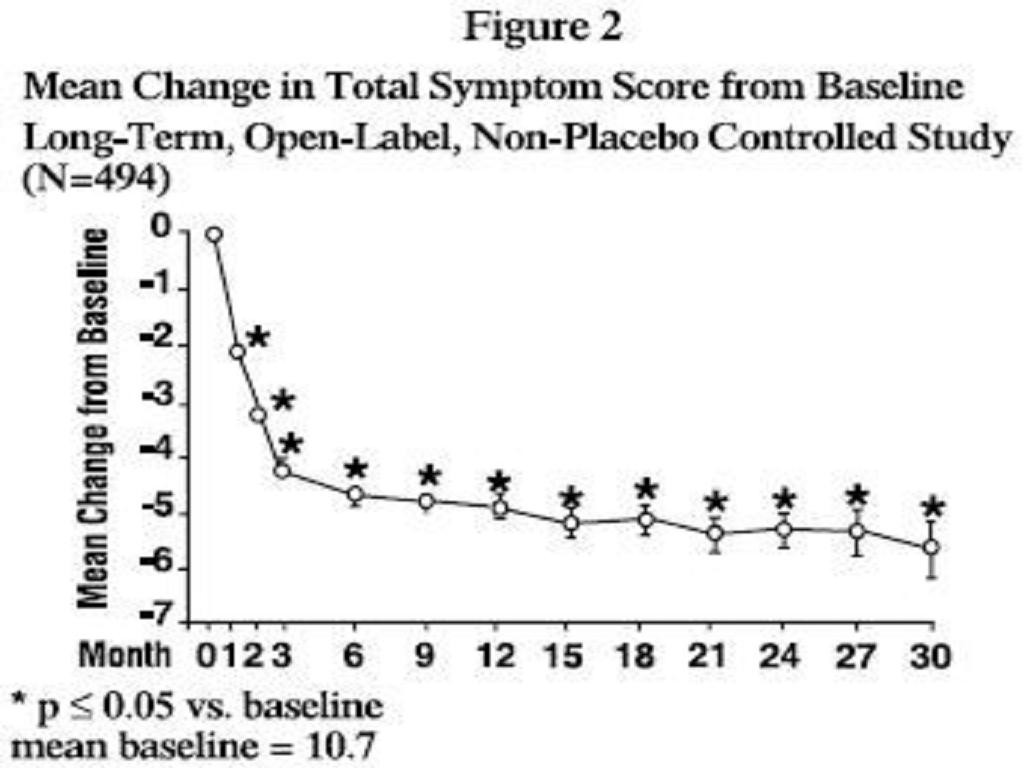
PHARMACODYNAMICS
Benign Prostatic Hyperplasia (BPH)Symptom Score (Range 027)Peak Flow Rate (mL/Sec)NMean BaselineMean Change (%)NMean BaselineMean Change (%)*Study 1 (10 mg)*Titration to fixed dose (12 wks)PlaceboTerazosinStudy 2 (2, 5, 10, 20 mg)Titration to response (24 wks)PlaceboTerazosinStudy 3 (1, 2, 5, 10 mg)Titration to response (24 wks)PlaceboTerazosin

Normotensive Patients DBP90 mm HgHypertensive Patients DBP > 90 mm HgGroupNMean ChangeNMean Change*****
Hypertension
PHARMACOKINETICS
INDICATIONS & USAGE
TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Syncope and "First-Dose" EffectCLINICAL PHARMACOLOGY
Priapism
PRECAUTIONS: Information for Patients
PRECAUTIONS
GeneralProstatic Cancer
Intraoperative Floppy Iris Syndrome (IFIS)
Orthostatic Hypotension
WARNINGS
INFORMATION FOR PATIENTS
Patient Package InsertLABORATORY TESTS
DRUG INTERACTIONS
Use with Other Drugs
DOSAGE AND ADMINISTRATION
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
Benign Prostatic HyperplasiaPRECAUTIONS
BENIGN PROSTATIC HYPERPLASIABody SystemTerazosin (N=636)Placebo (N=360)*BODY AS A WHOLE*CARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC AND NUTRITIONAL DISORDERSNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM
BENIGN PROSTATIC HYPERPLASIABody SystemTerazosin (N=636)Placebo (N=360)BODY AS A WHOLECARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM
Hypertension
HYPERTENSIONBody SystemTerazosin (N=859)Placebo (N=506)*BODY AS A WHOLE*CARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC AND NUTRITIONAL DISORDERSMUSCULOSKELETAL SYSTEMNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM
HYPERTENSIONBody SystemTerazosin (N=859)Placebo (N=506)BODY AS A WHOLECARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC AND NUTRITIONAL DISORDERSNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSES
Post-Marketing Experience
PRECAUTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Benign Prostatic Hyperplasia
Initial Dose
Subsequent Doses
Use With Other Drugs
PRECAUTIONS
Hypertension
Initial Dose
Subsequent Doses
Use With Other Drugs
HOW SUPPLIED
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
-
● a weak or interrupted stream when urinating
-
● a feeling that you cannot empty your bladder completely
-
● a feeling of delay when you start to urinate
-
● a need to urinate often, especially at night, or
-
● a feeling that you must urinate right away.
-
● Program of monitoring or "Watchful Waiting". Some men have an enlarged prostate gland, but no symptoms, or symptoms that are not bothersome. If this applies, you and your doctor may decide on a program of monitoring including regular checkups, instead of medication or surgery.
-
● Medication. There are different kinds of medication used to treat BPH. Your doctor has prescribed terazosin hydrochloride for you. See "What Terazosin Hydrochloride Does to Treat BPH" below.
-
● Surgery. Some patients may need surgery. Your doctor can describe several different surgical procedures to treat BPH. Which procedure is best depends on your symptoms and medical condition.
-
● Terazosin hydrochloride helps relieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
-
● If terazosin hydrochloride is helping you, you should notice an effect on your particular symptoms in 2 to 4 weeks of starting to take the medication.
-
● Even though you take terazosin hydrochloride and it may help you, terazosin hydrochloride may not prevent the need for surgery in the future.
-
● You should see an effect on your symptoms in 2 to 4 weeks. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular check-ups.
-
● Your doctor has prescribed terazosin hydrochloride for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking terazosin hydrochloride. Terazosin hydrochloride is not a treatment for prostate cancer.
-
● About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that terazosin hydrochloride does not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
-
● You will start with a 1 mg dose of terazosin hydrochloride. Then the dose will be increased as your body gets used to the effect of the medication.
-
● Other side effects you could have while taking terazosin hydrochloride include drowsiness, blurred or hazy vision, nausea, or "puffiness" of the feet or hands. Discuss any unexpected effects you notice with your doctor.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Terazosin HydrochlorideTerazosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!