Tears lubricant
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Tears lubricant Other information
- Inactive ingredients
- Package principal display panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Glycerin 0.2%
Hypromellose 0.2%
Polyethylene Glycol 400 1%
Purpose
Lubricant
Lubricant
Lubricant
Use
- for the temporary relief of burning and irritation due to dryness of the eye
- for protection against further irritation
Warnings
When using this product
- remove contact lenses before using
- do not use if this solution changes color or become cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye lasts
- condition worsens or lasts more than 72 hours
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Put 1 to 2 drops in the affected eye(s) up to 4 times daily
- children under 6 years of age: ask a doctor
Tears lubricant Other information
-
store between 15 ° to 25 ° C (59 ° F to 77 ° F)
Inactive ingredients
Ascorbic acid, benzalkonium chloride, boric acid, dextrose, magnesium chloride, potassium chloride, purified water, sodium borate, sodium chloride, sodium citrate, sodium lactate
Package principal display panel
Tears eye drop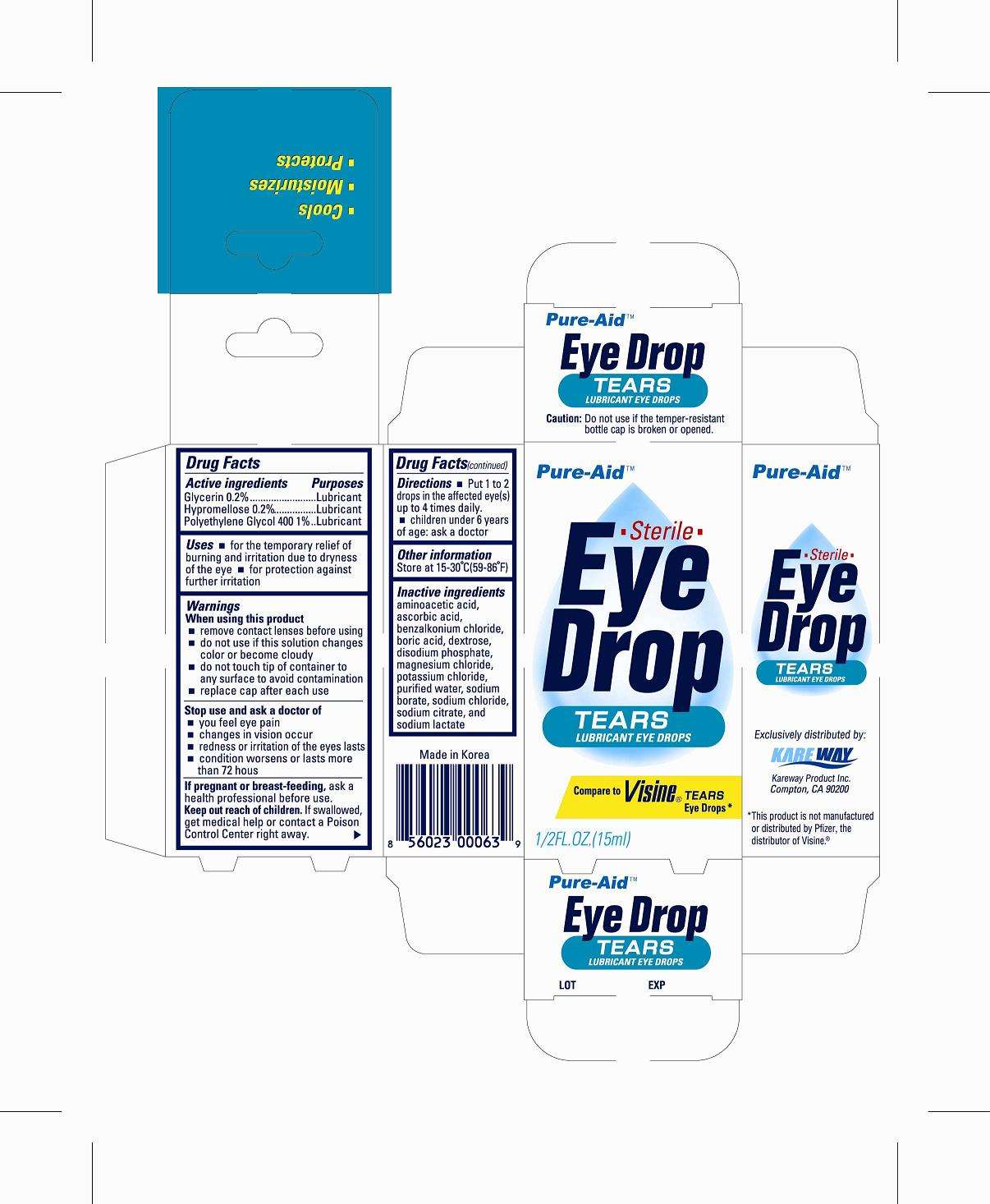
Tears lubricantglycerin, hypromelloses, polyethylene glycol 400 LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||