TE-Pads
ZO Skin Health
Lifetech Resources, LLC
TE-PadsOffects
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- TE-Pads Uses
- Warnings
- Directions
- TE-Pads Other information
- Inactive ingredients
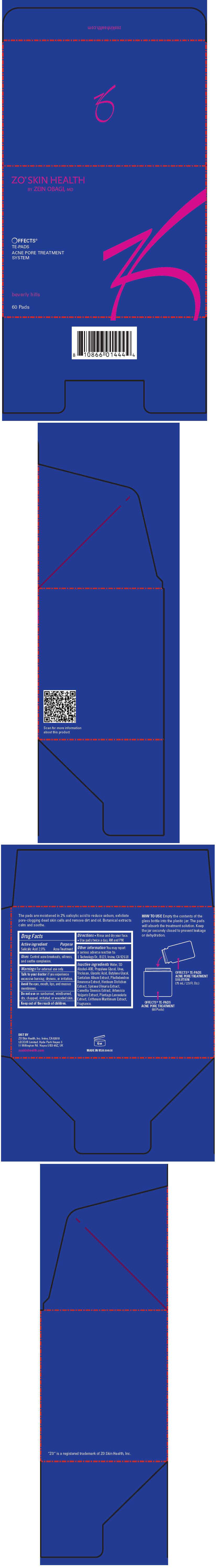
- PRINCIPAL DISPLAY PANEL - 60 Pad Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Salicylic Acid 2.0%
Purpose
Acne Treatment
TE-Pads Uses
Control acne breakouts, oiliness, and soothe complexion.
Warnings
For external use only.
Talk to your doctor if you experience excessive burning, dryness, or irritation.
Avoid the eyes, mouth, lips, and mucous membranes.
Do not use on sunburned, windburned, dry, chapped, irritated, or wounded skin.
Keep out of the reach of children.
Directions
- Rinse and dry your face.
- Use pads twice a day, AM and PM.
TE-Pads Other information
You may report a serious adverse reaction to: 1 Technology Dr., B123, Irvine, CA 92618
Inactive ingredients
Water, SD Alcohol-40B, Propylene Glycol, Urea, Triclosan, Glycolic Acid, Butylene Glycol, Santalum Album Extract, Phellodendron Amurense Extract, Hordeum Distichon Extract, Spiraea Ulmaria Extract, Camellia Sinensis Extract, Artemisia Vulgaris Extract, Plantago Lanceolata Extract, Crithmum Maritimum Extract, Fragrance.
DIST BY
ZO Skin Health, Inc. Irvine, CA 92618
LECEUR Limited. Hyde Park Hayes 3
11 Millington Rd. Hayes UB3 4AZ, UK
PRINCIPAL DISPLAY PANEL - 60 Pad Carton
ZO® SKIN HEALTH
BY ZEIN OBAGI, MD
OFFECTS
®
TE-PADS
ACNE PORE TREATMENT
SYSTEM
beverly hills
60 Pads
TE-PadsSALICYLIC ACID SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||