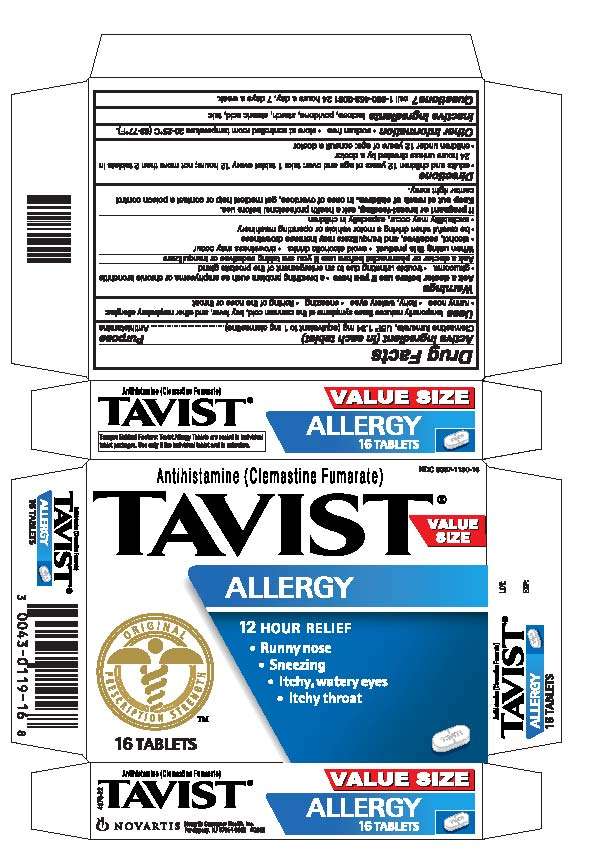
TAVIST
Novartis Consumer Health, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- TAVIST Uses
- Warnings
- Ask Doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- If pregnant or breast-feeding,
- Keep Out of Reach of Children
- Directions
- TAVIST Other information
- Inactive ingredients
- Questions or comments?
- Principal Display
FULL PRESCRIBING INFORMATION
Active ingredient
Clemastine fumarate, USP 1.34 mg (equivalent to 1 mg clemastine)
Purpose
Antihistamine
TAVIST Uses
temporarily reduces these symptoms of the common cold, hay fever and other respiratory allergies:
• runny nose
• itchy, watery eyes
• sneezing
• itching of the nose or throat
Warnings
Ask Doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
taking sedative or tranquillizers
When using this product
- avoid alcoholic drinks
- drowsiness may occur
- alcohol, sedative, and tranquillizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding,
ask a health professional before use.
Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and over: take 1 tablet every 12 hours; not more than 2 tablets in 24 hours unless direction by a doctor
- children under 12 years of age: consult a doctor
TAVIST Other information
- sodium free
- store at controlled room temperature 20-25°C (68-77°F)
Inactive ingredients
lactose, povidone, starch, stearic acid, talc
Questions or comments?
call 1-800-452-0051
Dist. by:
Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0602
Principal Display
TAVISTClemastine fumarate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!