Tangerin Whitening Hand
TONYMOLY CO., LTD.
TONYMOLY CO., LTD.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- TANGERIN WHITENING HAND INDICATIONS AND USAGE
- TANGERIN WHITENING HAND DOSAGE AND ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
Active Ingredient: Glycerin 20%
INACTIVE INGREDIENT
Inactive Ingredients:
Water, Vegetable Oil, Cyclopentasiloxane, Cyclohexasiloxane, Caprylic/Capric Triglyceride, Tribehenin PEG-20 Esters, Octyldodecanol, Citrus Aurantium Dulcis (Orange) Fruit Extract (2%)&Butylene Glycol (5%)& Water(93%), Butyrospermum Parkii(Shea Butter), Arbutin, Dipropylene Glycol, Citrus Medica Limonum (Lemon) Fruit Extract(2%) & Butylene Glycol(5%)&Water(93%), Dimethicone, Citrus Junos Fruit Extract(2%)& Propylene Glycol(10%) & Water(88%), Glyceryl Stearate, Fragrance, Phenoxyethanol, Methylparaben, Propylparaben, Xanthan Gum, Carbomer, Triethanolamine, CI 19140, CI 17200
PURPOSE
PURPOSE: Skin Protectant
WARNINGS
Warnings:
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of having problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children:
Keep out of reach of babies and children
TANGERIN WHITENING HAND INDICATIONS AND USAGE
INDICATIONS AND USAGE: Clean the hands, remove moist, apply sufficient amount on the hands. Could use on elbows and knees.
TANGERIN WHITENING HAND DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION: Take an adequate amount of this product.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
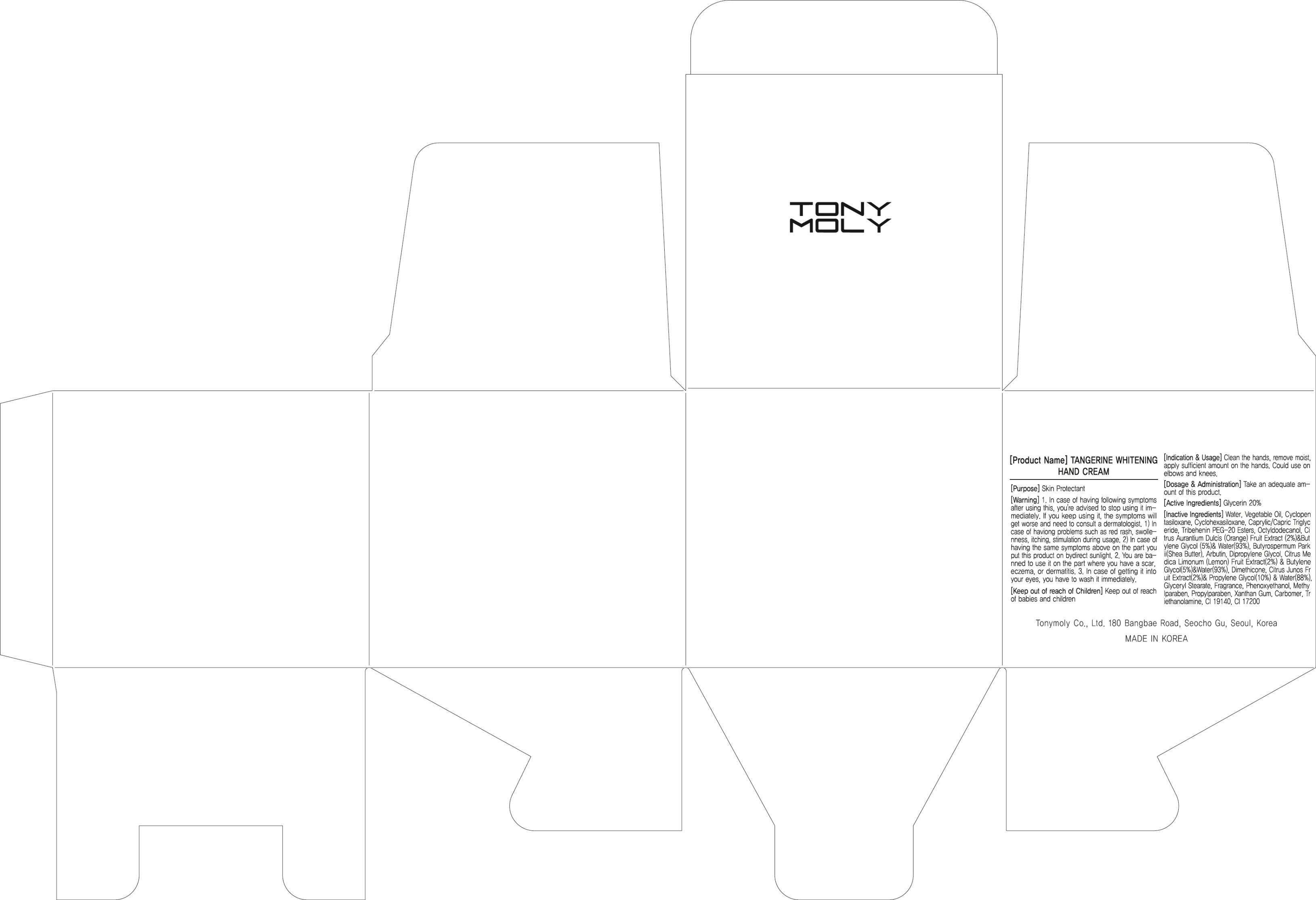
Tangerin Whitening HandGlycerin CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||