Tamoxifen Citrate
Bryant Ranch Prepack
Bryant Ranch Prepack
TAMOXIFEN CITRATE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- TAMOXIFEN CITRATE DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- CLINICAL STUDIES:
- TAMOXIFEN CITRATE INDICATIONS AND USAGE:
- TAMOXIFEN CITRATE CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- TAMOXIFEN CITRATE ADVERSE REACTIONS:
- OVERDOSAGE:
- TAMOXIFEN CITRATE DOSAGE AND ADMINISTRATION:
- MEDICATION GUIDE
- Tamoxifen Citrate 20mg Tablet
FULL PRESCRIBING INFORMATION
DESCRIPTION:
Tamoxifen citrate tablets, USP, a nonsteroidal antiestrogen, are for oral administration. Chemically, tamoxifen is the trans-isomer of a triphenylethylene derivative. The chemical name is (Z)2-[4-(1,2-diphenyl-1-butenyl) phenoxy]-N, N-dimethylethanamine 2 hydroxy-1,2,3- propanetricarboxylate (1:1). The structural formula is as follows:
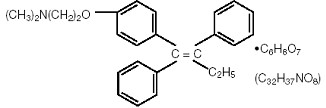
C H NO.C H O Molecular Weight: 563.62 26 29 6 8 7
The pKa' is 8.85, the equilibrium solubility in water at 37°C is 0.5 mg/mL and in 0.02 N HCl at 37°C, it is 0.2 mg/mL.
: Each tablet contains 15.2 mg of tamoxifen citrate which is equivalent to 10 mg of tamoxifen. 10 mg Tablets
: Each tablet contains 30.4 mg of tamoxifen citrate which is equivalent to 20 mg of tamoxifen. 20 mg Tablets
Each tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
CLINICAL PHARMACOLOGY:
Tamoxifen citrate is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Tamoxifen inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, tamoxifen appears to exert its antitumor effects by binding the estrogen receptors.
In cytosols derived from human breast adenocarcinomas, tamoxifen competes with estradiol for estrogen receptor protein.
CLINICAL STUDIES:
INDICATIONS AND USAGE:
CONTRAINDICATIONS:
Tamoxifen citrate tablets are contraindicated in patients with known hypersensitivity to the drug or any of its ingredients.
WARNINGS:
PRECAUTIONS:
General:
Decreases in platelet counts, usually to 50,000-100,000/mm , infrequently lower, have been occasionally reported in patients taking tamoxifen for breast cancer. In patients with significant thrombocytopenia, rare hemorrhagic episodes have occurred, but it is uncertain if these episodes are due to tamoxifen therapy. Leukopenia has been observed, sometimes in association with anemia and/or thrombocytopenia. There have been rare reports of neutropenia and pancytopenia in patients receiving tamoxifen; this can sometimes be severe. 3
In the NSABP P-1 trial, 6 women on tamoxifen citrate and 2 on placebo experienced grade 3-4 drops in platelet counts (≤ 50,000/mm ). 3
Information for Patients:
Patients should be instructed to read the Medication Guide supplied as required by law when tamoxifen citrate is dispensed. The complete text of the Medication Guide is reprinted at the end of this document.
Laboratory Tests:
Periodic complete blood counts, including platelet counts, and periodic liver function tests should be obtained.
During the ATAC trial, more patients receiving anastrozole were reported to have an elevated serum cholesterol compared to patients receiving tamoxifen citrate (9% versus 3.5%, respectively).
Drug Interactions:
When tamoxifen is used in combination with coumarin-type anticoagulants, a significant increase in anticoagulant effect may occur. Where such coadministration exists, careful monitoring of the patient's prothrombin time is recommended.
In the NSABP P-1 trial, women who required coumarin-type anticoagulants for any reason were ineligible for participation in the trial (See CONTRAINDICATIONS).
There is an increased risk of thromboembolic events occurring when cytotoxic agents are used in combination with tamoxifen.
Tamoxifen reduced letrozole plasma concentrations by 37%. The effect of tamoxifen on metabolism and excretion of other antineoplastic drugs, such as cyclophosphamide and other drugs that require mixed function oxidases for activation, is not known. Tamoxifen and N-desmethyl tamoxifen plasma concentrations have been shown to be reduced when coadministered with rifampin or aminoglutethimide. Induction of CYP3A4-mediated metabolism is considered to be the mechanism by which these reductions occur; other CYP3A4 inducing agents have not been studied to confirm this effect.
One patient receiving tamoxifen with concomitant phenobarbital exhibited a steady state serum level of tamoxifen lower than that observed for other patients (i.e., 26 ng/mL vs. mean value of 122 ng/mL). However, the clinical significance of this finding is not known. Rifampin induced the metabolism of tamoxifen and significantly reduced the plasma concentrations of tamoxifen in 10 patients. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
Concomitant bromocriptine therapy has been shown to elevate serum tamoxifen and N-desmethyl tamoxifen.
Based on clinical and pharmacokinetic results from the anastrozole adjuvant trial, tamoxifen citrate should not be administered with anastrozole (see CLINICAL PHARMACOLOGY, Drug-Drug Interactions section).
Drug/Laboratory Testing Interactions:
During postmarketing surveillance, T elevations were reported for a few postmenopausal patients which may be explained by increases in thyroid-binding globulin. These elevations were not accompanied by clinical hyperthyroidism. 4
Variations in the karyopyknotic index on vaginal smears and various degrees of estrogen effect on Pap smears have been infrequently seen in postmenopausal patients given tamoxifen.
In the postmarketing experience with tamoxifen, infrequent cases of hyperlipidemias have been reported. Periodic monitoring of plasma triglycerides and cholesterol may be indicated in patients with pre-existing hyperlipidemias (See ADVERSE REACTIONS, Postmarketing Experience section).
Carcinogenesis:
A conventional carcinogenesis study in rats at doses of 5, 20, and 35 mg/kg/day (about one, three and seven-fold the daily maximum recommended human dose on a mg/m basis) administered by oral gavage for up to 2 years revealed a significant increase in hepatocellular carcinoma at all doses. The incidence of these tumors was significantly greater among rats administered 20 or 35 mg/kg/day (69%) compared to those administered 5 mg/kg/day (14%). In a separate study, rats were administered tamoxifen at 45 mg/kg/day (about nine-fold the daily maximum recommended human dose on a mg/m basis); hepatocellular neoplasia was exhibited at 3 to 6 months. 2 2
Granulosa cell ovarian tumors and interstitial cell testicular tumors were observed in two separate mouse studies. The mice were administered the trans and racemic forms of tamoxifen for 13 to 15 months at doses of 5, 20 and 50 mg/kg/day (about one-half, two and five-fold the daily recommended human dose on a mg/m basis). 2
Mutagenesis:
No genotoxic potential was found in a conventional battery of and tests with pro- and eukaryotic test systems with drug metabolizing systems. However, increased levels of DNA adducts were observed by P post-labeling in DNA from rat liver and cultured human lymphocytes. Tamoxifen also has been found to increase levels of micronucleus formation in human lymphoblastoid cell line (MCL-5). Based on these findings, tamoxifen is genotoxic in rodent and human MCL-5 cells. in vivo in vitro 32 in vitro
Impairment of Fertility:
Tamoxifen produced impairment of fertility and conception in female rats at doses of 0.04 mg/kg/day (about 0.01-fold the daily maximum recommended human dose on a mg/m basis) when dosed for two weeks prior to mating through day 7 of pregnancy. At this dose, fertility and reproductive indices were markedly reduced with total fetal mortality. Fetal mortality was also increased at doses of 0.16 mg/kg/day (about 0.03-fold the daily maximum recommended human dose on a mg/m basis) when female rats were dosed from days 7-17 of pregnancy. Tamoxifen produced abortion, premature delivery and fetal death in rabbits administered doses equal to or greater than 0.125 mg/kg/day (about 0.05-fold the daily maximum recommended human dose on a mg/m basis). There were no teratogenic changes in either rats or rabbits. 2 2 2
Pregnancy Category D:
See WARNINGS.
Nursing Mothers:
Tamoxifen has been reported to inhibit lactation. Two placebo-controlled studies in over 150 women have shown that tamoxifen significantly inhibits early postpartum milk production. In both studies tamoxifen was administered within 24 hours of delivery for between 5 and 18 days. The effect of tamoxifen on established milk production is not known.
There are no data that address whether tamoxifen is excreted into human milk. If excreted, there are no data regarding the effects of tamoxifen in breast milk on the breastfed infant or breastfed animals. However, direct neonatal exposure of tamoxifen to mice and rats (not via breast milk) produced 1) reproductive tract lesions in female rodents (similar to those seen in humans after intrauterine exposure to diethylstilbestrol) and 2) functional defects of the reproductive tract in male rodents such as testicular atrophy and arrest of spermatogenesis.
It is not known if tamoxifen citrate is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from tamoxifen, women taking tamoxifen should not breast feed.
Pediatric Use:
In adults treated with tamoxifen, an increase in incidence of uterine malignancies, stroke and pulmonary embolism has been noted (see BOXED WARNING and CLINICAL PHARMACOLOGY, Clinical Studies, McCune-Albright Syndrome subsection). The safety and efficacy of tamoxifen for girls aged two to 10 years with McCune-Albright Syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy for girls have not been established.
Geriatric Use:
In the NSABP P-1 trial, the percentage of women at least 65 years of age was 16%. Women at least 70 years of age accounted for 6% of the participants. A reduction in breast cancer incidence was seen among participants in each of the subsets: A total of 28 and 10 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. Across all other outcomes, the results in this subset reflect the results observed in the subset of women at least 50 years of age. No overall differences in tolerability were observed between older and younger patients (See CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women section).
In the NSABP B-24 trial, the percentage of women at least 65 years of age was 23%. Women at least 70 years of age accounted for 10% of participants. A total of 14 and 12 invasive breast cancers were seen among participants 65 and older in the placebo and tamoxifen groups, respectively. This subset is too small to reach any conclusions on efficacy. Across all other endpoints, the results in this subset were comparable to those of younger women enrolled in this trial. No overall differences in tolerability were observed between older and younger patients.
ADVERSE REACTIONS:
Adverse reactions to tamoxifen are relatively mild and rarely severe enough to require discontinuation of treatment in breast cancer patients.
Continued clinical studies have resulted in further information which better indicates the incidence of adverse reactions with tamoxifen as compared to placebo.
OVERDOSAGE:
Signs observed at the highest doses following studies to determine LD in animals were respiratory difficulties and convulsions. 50
Acute overdosage in humans has not been reported. In a study of advanced metastatic cancer patients which specifically determined the maximum tolerated dose of tamoxifen in evaluating the use of very high doses to reverse multidrug resistance, acute neurotoxicity manifested by tremor, hyperreflexia, unsteady gait and dizziness were noted. These symptoms occurred within 3-5 days of beginning tamoxifen and cleared within 2-5 days after stopping therapy. No permanent neurologic toxicity was noted. One patient experienced a seizure several days after tamoxifen was discontinued and neurotoxic symptoms had resolved. The causal relationship of the seizure to tamoxifen therapy is unknown. Doses given in these patients were all greater than 400 mg/m loading dose, followed by maintenance doses of 150 mg/m of tamoxifen given twice a day. 2 2
In the same study, prolongation of the QT interval on the electrocardiogram was noted when patients were given doses higher than 250 mg/m loading dose, followed by maintenance doses of 80 mg/m of tamoxifen given twice a day. For a woman with a body surface area of 1.5 m the minimal loading dose and maintenance doses given at which neurological symptoms and QT changes occurred were at least 6 fold higher in respect to the maximum recommended dose. 2 2 2
No specific treatment for overdosage is known; treatment must be symptomatic.
DOSAGE AND ADMINISTRATION:
For patients with breast cancer, the recommended daily dose is 20-40 mg. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
In three single agent adjuvant studies in women, one 10 mg tamoxifen citrate tablet was administered two (ECOG and NATO) or three (Toronto) times a day for two years. In the NSABP B-14 adjuvant study in women with node-negative breast cancer, one 10 mg tamoxifen citrate tablet was given twice a day for at least 5 years. Results of the B-14 study suggest that continuation of therapy beyond five years does not provide additional benefit (see CLINICAL PHARMACOLOGY). In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy. There was no indication that doses greater than 20 mg per day were more effective. Current data from clinical trials support 5 years of adjuvant tamoxifen therapy for patients with breast cancer.
MEDICATION GUIDE
Tamoxifen Citrate Tablets, USP
(ta-MOX-I-fen)
Written for women who use tamoxifen to lower their high chance of getting breast cancer or who have ductal carcinoma in situ (DCIS)
This Medication Guide discusses only the use of tamoxifen to lower the chance of getting breast cancer in high-risk women and in women treated for DCIS.
People taking tamoxifen breast cancer have different benefits and different decisions to make than high-risk women or women with ductal carcinoma in situ (DCIS) taking tamoxifen to reduce the chance of getting breast cancer. If you already have breast cancer, talk with your doctor about how the benefits of treating breast cancer with tamoxifen compare to the risks that are described in this document. to treat
Why should I read this Medication Guide?
This guide has information to help you decide whether to use tamoxifen to lower your chance of getting breast cancer.
. Your doctor has a special computer program or hand-held calculator to tell if you are in the high-risk group. If you have DCIS and have been treated with surgery and radiation therapy, your doctor may prescribe tamoxifen to decrease your chance of getting invasive (spreading) breast cancer. You and your doctor should talk about whether the possible benefit of tamoxifen in lowering your high chance of getting breast cancer is greater than its possible risks
Read this guide carefully before you start tamoxifen. It is important to read the information you get each time you get more medicine. There may be something new. This guide does not tell you everything about tamoxifen and does take the place of talking with your doctor. not
Only you and your doctor can determine if tamoxifen is right for you.
What is the most important information I should know about using tamoxifen to reduce the chance of getting breast cancer?
Tamoxifen is a prescription medicine that is like estrogen (female hormone) in some ways and different in other ways. In the breast, tamoxifen can block estrogen’s effects. Because it does this, tamoxifen may block the growth of breast cancers that need estrogen to grow (cancers that are estrogen- or progesterone-receptor positive).
Tamoxifen can lower the chance of getting breast cancer in women with a higher than normal chance of getting breast cancer in the next five years (high-risk women) and women with DCIS.
. Because high-risk women don’t have cancer yet, it is important to think carefully about whether the possible benefit of tamoxifen in lowering the chance of getting breast cancer is greater than its possible risks
This Medication Guide reviews the risks and benefits of using tamoxifen to reduce the chance of getting breast cancer in high-risk women and women with DCIS. This guide does discuss the special benefits and decisions for people who already have breast cancer. not
Why do women and men use tamoxifen?
Tamoxifen has more than one use. Tamoxifen is used:
- of getting breast cancer in women with a higher than normal chance of getting breast cancer in the next 5 years (high-risk women). to lower the chance
- of getting invasive (spreading) breast cancer in women who had surgery and radiation for ductal carcinoma in situ (DCIS). DCIS means the cancer is only inside the milk ducts. to lower the chance
- breast cancer in women after they have finished early treatment. Early treatment can include surgery, radiation, and chemotherapy. Tamoxifen may keep the cancer from spreading to other parts of the body. It may also reduce the woman’s chance of getting a new breast cancer. to treat
- in women and men, breast cancer that has spread to other parts of the body (metastatic breast cancer). to treat
This guide talks only about using tamoxifen to lower the chance of getting breast cancer (#1 and #2 above).
What are the benefits of tamoxifen to lower the chance of getting breast cancer in high-risk women and in women treated for DCIS?
- A large US study looked at and compared the ones who took tamoxifen for 5 years with others who took a pill without tamoxifen (placebo). High-risk women were defined as women who have a 1.7% or greater chance of getting breast cancer in the next 5 years, based on a special computer program. In this study: high-risk women
- Out of every 1,000 high-risk women , each year about 7 got breast cancer. who took a placebo
- Out of every 1,000 high-risk women , each year about 4 got breast cancer. who took tamoxifen
- The study showed that on average, high-risk women who took tamoxifen lowered their chances of getting breast cancer by 44%, from 7 in 1,000 to 4 in 1,000.
Another US study looked at and compared those who took tamoxifen for 5 years with others who took a placebo. In this study: women with DCIS
- Out of every 1,000 women with DCIS , each year about 17 got breast cancer. who took placebo
- Out of every 1,000 women with DCIS , each year about 10 got breast cancer. who took tamoxifen
The study showed that on average, women with DCIS who took tamoxifen lowered their chances of getting invasive (spreading) breast cancer by 43%, from 17 in 1,000 to 10 in 1,000.
. We do not know what the benefits will be for any one woman who takes tamoxifen citrate to reduce her chance of getting breast cancer. These studies do not mean that taking tamoxifen will lower your personal chance of getting breast cancer
What are the risks of tamoxifen?
In the studies described under “What are the benefits of tamoxifen?”, the high-risk women who took tamoxifen citrate got certain side effects at a higher rate than those who took a placebo. Some of these side effects can cause death.
In one study, in women who still had their uterus
- Out of every 1,000 women who took a placebo, each year 1 got endometrial cancer (cancer of the lining of the uterus) and none got uterine sarcoma (cancer of the body of the uterus).
- Out of every 1,000 women who took tamoxifen, each year 2 got endometrial cancer and fewer than 1 got uterine sarcoma.
These results show that, on average, in high-risk women , tamoxifen citrate doubled the chance of getting endometrial cancer from 1 in 1,000 to 2 in 1,000, and it increased the chance of getting uterine sarcoma. . We do not know what this risk will be for any one woman. The risk is different for women who no longer have their uterus. who still had their uterus This does not mean that taking tamoxifen will double your personal chance of getting endometrial cancer or increase your chance of getting uterine sarcoma
. In some cases, women died from these effects. For all women in this study, taking tamoxifen increased the risk of having a blood clot in their lungs or veins, or of having a stroke
. (See “What are the possible side effects of tamoxifen?” for more details about side effects.) Tamoxifen increased the risk of getting cataracts (clouding of the lens of the eye) or needing cataract surgery
What don’t we know about taking tamoxifen citrate to reduce the chance of getting breast cancer?
We don’t know
- if tamoxifen lowers the chance of getting breast cancer in women who have abnormal breast cancer genes (BRCA1 and BRCA2)
- if taking tamoxifen for 5 years reduces the number of breast cancers a woman will get in her lifetime or if it only delays some breast cancers
- if tamoxifen helps a woman live longer
- the effects of taking tamoxifen with hormone replacement therapy (HRT), birth control pills, or androgens (male hormones)
- the benefits of taking tamoxifen if you are less than 35 years old
Studies are being done to learn more about the long-term benefits and risks of using tamoxifen to reduce the chance of getting breast cancer.
What are the possible side effects of tamoxifen?
The most common side effect of tamoxifen is hot flashes. This is not a sign of a serious problem.
The next most common side effect is vaginal discharge. If the discharge is bloody, it could be a sign of a serious problem. [See “Changes in the lining (endometrium) or body of your uterus”
below.]
Less common but serious side effects of tamoxifen are listed below. These can occur at any time. Call your doctor right away if you have any signs of side effects listed below:
- . These changes may mean serious problems are starting, including cancer of the uterus. The signs of changes in the uterus are: Changes in the lining (endometrium) or body of your uterus
- Vaginal bleeding or bloody discharge that could be a rusty or brown color. You should call your doctor even if only a small amount of bleeding occurs.
- Change in your monthly bleeding, such as in the amount or timing of bleeding or increased clotting.
- Pain or pressure in your pelvis (below your belly button).
- . These can cause serious problems, including death. You may get clots up to 2-3 months after you stop taking tamoxifen citrate. The signs of blood clots are: Blood clots in your veins or lungs
- sudden chest pain, shortness of breath, coughing up blood
- pain, tenderness, or swelling in one or both of your legs
- . Stroke can cause serious medical problems, including death. The signs of stroke are: Stroke
- sudden weakness, tingling, or numbness in your face, arm or leg, especially on one side of your body
- sudden confusion, trouble speaking or understanding
- sudden trouble seeing in one or both eyes
- sudden trouble walking, dizziness, loss of balance or coordination
- sudden severe headache with no known cause
- . The sign of these problems is slow blurring of your vision. Cataracts or increased chance of needing cataract surgery
- . The signs of liver problems include lack of appetite and yellowing of your skin or whites of your eyes. Liver problems, including jaundice
These are not all the possible side effects of tamoxifen. For a complete list, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Who should not take tamoxifen?
Do not take tamoxifen for any reason if you
- . It takes about 2 months to clear tamoxifen from your body. To be sure you are not pregnant, you can start taking tamoxifen while you are having your menstrual period. Or, you can take a pregnancy test to be sure you are not pregnant before you begin. Are pregnant or plan to become pregnant while taking tamoxifen or during the 2 months after you stop taking tamoxifen. Tamoxifen may harm your unborn baby
- . We do not know if tamoxifen can pass through your milk and harm your baby. Are breast feeding
- or to any of its inactive ingredients. Have had an allergic reaction to tamoxifen
If you get pregnant while taking tamoxifen, stop taking it right away and contact your doctor. Tamoxifen may harm your unborn baby.
Do not take tamoxifen to lower your chance of getting breast cancer if
- You ever had a blood clot that needed medical treatment.
- You are taking medicines to thin your blood, like warfarin, (also called Coumadin *). ®
- Your ability to move around is limited for most of your waking hours.
- You are at risk for blood clots. Your doctor can tell you if you are at high risk for blood clots.
- You do not have a higher than normal chance of getting breast cancer. Your doctor can tell you if you are a high-risk woman.
How should I take tamoxifen?
- Swallow the tablet(s) whole, with water or another non-alcoholic liquid. You can take tamoxifen with or without food. Take your medicine every day. It may be easier to remember if you take it at the same time each day.
- If you forget a dose, take it when you remember, then take the next dose as usual. If it is almost time for your next dose or you remember at your next dose, do not take extra tablets to make up the missed dose.
- Take tamoxifen for 5 years, unless your doctor tells you otherwise.
What should I avoid while taking tamoxifen?
- . Tamoxifen can stop hormonal birth control methods from working. Hormonal methods include birth control pills, patches, injections, rings and implants. Therefore, while taking tamoxifen, use birth control methods that don’t use hormones, such as condoms, diaphragms with spermicide, or plain IUD’s. If you get pregnant, stop taking tamoxifen right away and call your doctor. Do not become pregnant while taking tamoxifen or for 2 months after you stop
- . We do not know if tamoxifen can pass through your milk and if it can harm the baby. Do not breast feed
What should I do while taking tamoxifen?
- Have regular gynecology check-ups (“female exams”), breast exams and mammograms. Your doctor will tell you how often. These will check for signs of breast cancer and cancer of the endometrium (lining of the uterus). Because tamoxifen does not prevent all breast cancers, and you may get other types of cancers, you need these exams to find any cancers as early as possible.
- Because tamoxifen can cause serious side effects, pay close attention to your body. Signs you should look for are listed in “What are the possible side effects of tamoxifen?”
- Tell all of the doctors that you see that you are taking tamoxifen.
- Tell your doctor right away if you have any new breast lumps.
General information about the safe and effective use of tamoxifen.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Your doctor has prescribed tamoxifen only for you. Do not give it to other people, even if they have a similar condition, because it may harm them. Do not use it for a condition for which it was not prescribed.
This Medication Guide is a summary of information about tamoxifen for women who use tamoxifen to lower their high chance of getting breast cancer or who have DCIS. If you want more information about tamoxifen, ask your doctor or pharmacist. They can give you information about tamoxifen that is written for health professionals. For more information about tamoxifen or breast cancer, call 1-800-272-5525.
Ingredients: tamoxifen citrate, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
*Coumadin is a registered trademark of Bristol-Myers Squibb Pharmaceuticals. ®
This Medication Guide has been approved by the US Food and Drug Administration.
Manufactured By: Corona, CA 92880 USA Distributed By: Corona, CA 92880 USA
Watson Laboratories, INC.
Watson Pharma, Inc.
Revised: October 2010
Tamoxifen Citrate 20mg Tablet

Tamoxifen CitrateTamoxifen Citrate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||