Tamiflu
These highlights do not include all the information needed to use TAMIFLU safely and effectively. See full prescribing information for TAMIFLU. TAMIFLU® (oseltamivir phosphate) capsules, for oral use TAMIFLU® (oseltamivir phosphate) for oral suspension Initial U.S. Approval: 1999
FULL PRESCRIBING INFORMATION: CONTENTS*
- RECENT MAJOR CHANGES
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- TAMIFLU CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- TAMIFLU ADVERSE REACTIONS
- DRUG INTERACTIONS
- USE IN SPECIFIC POPULATIONS
- OVERDOSAGE
- TAMIFLU DESCRIPTION
- CLINICAL PHARMACOLOGY
- NONCLINICAL TOXICOLOGY
- CLINICAL STUDIES
- HOW SUPPLIED
- INFORMATION FOR PATIENTS
- SPL UNCLASSIFIED
- SPL PATIENT PACKAGE INSERT
- SPL PATIENT PACKAGE INSERT
- SPL UNCLASSIFIED
- SPL UNCLASSIFIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
RECENT MAJOR CHANGES
| Indications and Usage (1.1) | 12/2012 |
| Dosage and Administration (2.1, 2.2, 2.3, 2.4. 2.8) | 12/2012 |
| Warnings and Precautions (5.4) | 12/2012 |
INDICATIONS & USAGE
TAMIFLU is an influenza neuraminidase inhibitor indicated for:
- Treatment of acute, uncomplicated influenza in patients 2 weeks of age and older who have been symptomatic for no more than 2 days. (1.1)
- Prophylaxis of influenza in patients 1 year and older. (1.2)
Important Limitations of Use:
- Efficacy not established in patients who begin therapy after 48 hours of symptoms. (1.3)
- Not a substitute for annual influenza vaccination. (1.3)
- No evidence of efficacy for illness from agents other than influenza viruses types A and B. (1.3)
- Consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use. (1.3)
TAMIFLU is indicated for the treatment of acute, uncomplicated illness due to influenza infection in patients 2 weeks of age and older who have been symptomatic for no more than 2 days.
TAMIFLU is indicated for the prophylaxis of influenza in patients 1 year and older.
The following points should be considered before initiating treatment or prophylaxis with TAMIFLU:
- Efficacy of TAMIFLU in patients who begin treatment after 48 hours of symptoms has not been established.
- TAMIFLU is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
- There is no evidence for efficacy of TAMIFLU in any illness caused by agents other than influenza viruses types A and B.
- Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use TAMIFLU.
DOSAGE & ADMINISTRATION
Treatment of influenza (2.2)
- Adults and adolescents (13 years and older): 75 mg twice daily for 5 days
- Pediatric patients 1 to 12 years of age: Based on weight twice daily for 5 days
- Pediatric patients 2 weeks to less than 1 year of age: 3mg/kg twice daily for 5 days.
- Renally impaired adult patients (creatinine clearance 10-30 mL/min): Reduce to 75 mg once daily for 5 days (2.4)
Prophylaxis of influenza (2.3)
- Adults and adolescents (13 years and older): 75 mg once daily for at least 10 days-Community outbreak: 75 mg once daily for up to 6 weeks
- Pediatric patients 1 to 12 years of age: Based on weight once daily for 10 days-Community outbreak: Based on weight once daily for up to 6 weeks
- Renally impaired adult patients (creatinine clearance 10-30 mL/min): Reduce to 75 mg once every other day or 30 mg once daily (2.4)
Treatment with TAMIFLU should begin within 2 days of onset of symptoms of influenza or following close contact with an infected individual.
TAMIFLU may be taken with or without food [see Clinical Pharmacology (12.3)]. However, when taken with food, tolerability may be enhanced in some patients.
For patients who cannot swallow capsules, TAMIFLU for oral suspension is the preferred formulation. If the oral suspension product is not available, TAMIFLU capsules may be opened and mixed with sweetened liquids such as regular or sugar-free chocolate syrup, corn syrup, caramel topping, or light brown sugar (dissolved in water). If the appropriate strengths of TAMIFLU capsules are not available to mix with sweetened liquids and the oral suspension product is not available, then a pharmacist may compound an emergency supply of oral suspension from TAMIFLU 75 mg capsules [see Dosage and Administration (2.8)].
Adults and Adolescents (13 years of age and older)
The recommended oral dose of TAMIFLU for treatment of influenza in adults and adolescents 13 years and older is 75 mg twice daily for 5 days. TAMIFLU for oral suspension may be used by patients who cannot swallow a capsule (12.5 mL of TAMIFLU for oral suspension [6 mg/mL] delivers 75 mg) [see Dosage and Administration (2.1)].
Pediatric Patients (2 weeks to 12 years of age)
The recommended oral dose of TAMIFLU for treatment of influenza in pediatric patients 1 year to 12 years of age is shown in Table 1.
The recommended oral dose of TAMIFLU for treatment of influenza in pediatric patients 2 weeks to less than 1 year of age is 3 mg/kg twice daily for 5 days (shown in Table 1).
Adults and Adolescents (13 years of age and older)
The recommended oral dose of TAMIFLU for prophylaxis of influenza in adults and adolescents 13 years and older following close contact with an infected individual is 75 mg once daily for at least 10 days. The recommended dose for prophylaxis during a community outbreak of influenza is 75 mg once daily. Safety and efficacy have been demonstrated for up to 6 weeks in immunocompetent patients. The duration of protection lasts for as long as dosing is continued. Safety has been demonstrated for up to 12 weeks in immunocompromised patients. TAMIFLU for oral suspension may also be used by patients who cannot swallow a capsule (12.5 mL of TAMIFLU for oral suspension [6 mg/mL] delivers 75 mg).
Pediatric Patients (2 weeks to 12 years of age)
The recommended oral dose of TAMIFLU for prophylaxis of influenza in pediatric patients 1 to 12 years of age based on body weight is shown in Table 1. Prophylaxis in pediatric patients following close contact with an infected individual is recommended for 10 days. For prophylaxis in pediatric patients during a community outbreak of influenza, dosing may be continued for up to 6 weeks.
The safety and efficacy of TAMIFLU for prophylaxis of influenza have not been established in infants less than 1 year of age.
|
Weight (kg) |
Treatment Dosing for 5 days | Prophylaxis Dosing for 10 days | Volume of Oral Suspension (6 mg/mL) for each Dose* | Number of Bottles of Oral Suspension to Dispense | Number of Capsules and Strength to Dispense† | |
|---|---|---|---|---|---|---|
| Patients from 2 Weeks to less than 1 Year of Age | ||||||
| Any weight | 3 mg/kg twice daily | Not applicable‡ | 0.5 mL/kg§ | 1 bottle | Not applicable | |
| Patients 1 to 12 Years of Age Based on Body Weight | ||||||
| 15 kg or less | 30 mg twice daily | 30 mg once daily | 5 mL | 1 bottle | 10 Capsules 30 mg |
|
| 15.1 kg thru 23 kg | 45 mg twice daily | 45 mg once daily | 7.5 mL | 2 bottles | 10 Capsules 45 mg |
|
| 23.1 kg thru 40 kg | 60 mg twice daily | 60 mg once daily | 10 mL | 2 bottles | 20 Capsules 30 mg |
|
| 40.1 kg or more | 75 mg twice daily | 75 mg once daily | 12.5 mL¶ | 3 bottles | 10 Capsules 75 mg |





Data are available on plasma concentrations of oseltamivir carboxylate following various dosing schedules in patients with renal impairment [see Clinical Pharmacology (12.3)].
Treatment of Influenza
Dose adjustment is recommended for adult patients with creatinine clearance between 10 and 30 mL/min receiving TAMIFLU for the treatment of influenza. In these patients it is recommended that the dose be reduced to 75 mg of TAMIFLU once daily for 5 days. No recommended dosing regimens are available for patients with end-stage renal disease undergoing routine hemodialysis or continuous peritoneal dialysis treatment.
Prophylaxis of Influenza
For the prophylaxis of influenza, dose adjustment is recommended for adult patients with creatinine clearance between 10 and 30 mL/min receiving TAMIFLU. In these patients it is recommended that the dose be reduced to 75 mg of TAMIFLU every other day or 30 mg TAMIFLU every day. No recommended dosing regimens are available for patients undergoing routine hemodialysis and continuous peritoneal dialysis treatment with end-stage renal disease.
No dose adjustment is recommended for patients with mild or moderate hepatic impairment (Child-Pugh score ≤9) [see Clinical Pharmacology (12.3)].
No dose adjustment is required for geriatric patients [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3)].
It is recommended that TAMIFLU for oral suspension be constituted by the pharmacist prior to dispensing to the patient:
a)Tap the closed bottle several times to loosen the powder.b)Measure
55 mL
of water in a graduated cylinder.c)Add the total amount of water for constitution to the bottle and shake the closed bottle well for 15 seconds.d)Remove the child-resistant cap and push bottle adapter into the neck of the bottle.e)Close bottle with child-resistant cap tightly. This will assure the proper seating of the bottle adapter in the bottle and child-resistant status of the cap.
Label the bottle with instructions to Shake Well before each use.
The constituted TAMIFLU for oral suspension (6 mg/mL) should be used within 17 days of preparation when stored under refrigeration or within 10 days if stored at controlled room temperature; the pharmacist should write the date of expiration of the constituted suspension on a pharmacy label. The patient package insert and oral dispenser should be dispensed to the patient.
The following directions are provided for use only during emergency situations. These directions are not intended to be used if the FDA-approved, commercially manufactured TAMIFLU for oral suspension is readily available from wholesalers or the manufacturer.
Compounding an oral suspension with this procedure will provide one patient with enough medication for a 5-day course of treatment or a 10-day course of prophylaxis.
Commercially manufactured TAMIFLU for oral suspension (6 mg/mL) is the preferred product for pediatric and adult patients who have difficulty swallowing capsules or where lower doses are needed. In the event that TAMIFLU for oral suspension is not available, the pharmacist may compound a suspension (6 mg/mL) from TAMIFLU capsules 75 mg using one of these vehicles: Cherry Syrup (Humco®), Ora-Sweet® SF (sugar-free) (Paddock Laboratories), or simple syrup. Other vehicles have not been studied. This compounded suspension should not be used for convenience or when the FDA-approved TAMIFLU for oral suspension is commercially available.
First, determine the dose of TAMIFLU for the patients [see Dosage and Administration (2) ] then determine total volume of an oral suspension needed to be compounded based on Table 2.
| TAMIFLU Dose* | Total Volume to Compound per Patient (mL) | |
|---|---|---|
| 15 mg or less | 37.5 mL | |
| 30 mg | 75 mL | |
| 45 mg | 100 mL | |
| 60 mg | 125 mL | |
| 75 mg | 150 mL |

Second, determine the number of capsules and the amount of water and vehicle (Cherry Syrup, Ora-Sweet® SF, or simple syrup) that are needed to prepare the total volume (determined from Table 2: 37.5 mL, 75 mL, 100 mL, 125 mL, or 150 mL) of compounded oral suspension (6 mg/mL) (see Table 3).
| Total Volume of Compounded Oral Suspension to be Prepared | 37.5 mL | 75 mL | 100 mL | 125 mL | 150 mL |
|---|---|---|---|---|---|
| Number of TAMIFLU 75 mg Capsules* | 3 capsules (225mg oseltamivir) | 6 capsules (450 mg oseltamivir) | 8 capsules (600 mg oseltamivir) | 10 capsules (750 mg oseltamivir) | 12 capsules (900 mg oseltamivir) |
| Amount of Water | 2.5 mL | 5 mL | 7 mL | 8 mL | 10 mL |
|
Volume of Vehicle Cherry Syrup (Humco®) OR Ora-Sweet® SF (Paddock Laboratories) OR simple syrup |
34.5 mL | 69 mL | 91 mL | 115 mL | 137 mL |

Third, follow the procedure below for compounding the oral suspension (6 mg/mL) from TAMIFLU capsules 75 mg:
- Place the specified amount of water into a polyethyleneterephthalate (PET) or glass bottle (see Table 3).
- Carefully separate the capsule body and cap and pour the contents of the required number of TAMIFLU 75 mg capsules into the PET or glass bottle.
- Gently swirl the suspension to ensure adequate wetting of the TAMIFLU powder for at least 2 minutes.
- Slowly add the specified amount of vehicle to the bottle.
- Close the bottle using a child-resistant cap and shake well for 30 seconds to completely dissolve the active drug and to ensure homogeneous distribution of the dissolved drug in the resulting suspension. (Note: The active drug, oseltamivir phosphate, readily dissolves in the specified vehicles. The suspension is caused by inert ingredients of TAMIFLU capsules which are insoluble in these vehicles.)
- Put an ancillary label on the bottle indicating "Shake Well Before Use."
- Instruct the parent or caregiver that any unused suspension remaining in the bottle following completion of therapy must be discarded by either affixing an ancillary label to the bottle or adding a statement to the pharmacy label instructions.
- Place an appropriate expiration date on the label according to storage conditions below.
Storage of the Emergency Compounded Suspension
- Refrigeration: Stable for 5 weeks (35 days) when stored in a refrigerator at 2° to 8°C (36° to 46°F).
- Room Temperature: Stable for five days (5 days) when stored at room temperature, 25°C (77°F).
Note: The storage conditions are based on stability studies of compounded oral suspensions, using the above mentioned vehicles, which were placed in glass and polyethyleneterephthalate (PET) bottles. Stability studies have not been conducted with other vehicles or bottle types.
Place a pharmacy label on the bottle that includes the patient's name, dosing instructions, and drug name and any other required information to be in compliance with all State and Federal Pharmacy Regulations.
Dosing of the Compounded Suspension (6 mg/mL)
Refer to Dosage and Administration sections 2.2, 2.3, 2.4 and Table 1 for the proper dosing instructions for the pharmacy label.
DOSAGE FORMS & STRENGTHS
Capsules: 30 mg, 45 mg, 75 mg
- 30-mg capsules (30 mg free base equivalent of the phosphate salt): light yellow hard gelatin capsules. "ROCHE" is printed in blue ink on the light yellow body and "30 mg" is printed in blue ink on the light yellow cap.
- 45-mg capsules (45 mg free base equivalent of the phosphate salt): grey hard gelatin capsules. "ROCHE" is printed in blue ink on the grey body and "45 mg" is printed in blue ink on the grey cap.
- 75-mg capsules (75 mg free base equivalent of the phosphate salt): grey/light yellow hard gelatin capsules. "ROCHE" is printed in blue ink on the grey body and "75 mg" is printed in blue ink on the light yellow cap.
For Oral Suspension: 6 mg/mL (final concentration when constituted)
- White powder blend for constitution to a white tutti-frutti–flavored suspension. After constitution, each bottle delivers a usable volume of 60 mL of oral suspension equivalent to 360 mg oseltamivir base (6 mg/mL).
TAMIFLU CONTRAINDICATIONS
TAMIFLU is contraindicated in patients with known serious hypersensitivity to oseltamivir or any component of the product. Severe allergic reactions have included anaphylaxis and serious skin reactions including toxic epidermal necrolysis, Stevens-Johnson Syndrome, and erythema multiforme [see Warnings and Precautions (5.1)].
WARNINGS AND PRECAUTIONS
- Serious skin/hypersensitivity reactions such as Stevens-Johnson Syndrome, toxic epidermal necrolysis and erythema multiforme: Discontinue TAMIFLU and initiate appropriate treatment if allergic-like reactions occur or are suspected. (5.1)
- Neuropsychiatric events: Patients with influenza, including those receiving TAMIFLU, particularly pediatric patients, may be at an increased risk of confusion or abnormal behavior early in their illness. Monitor for signs of abnormal behavior. (5.2)
Cases of anaphylaxis and serious skin reactions including toxic epidermal necrolysis, Stevens-Johnson Syndrome, and erythema multiforme have been reported in postmarketing experience with TAMIFLU. TAMIFLU should be stopped and appropriate treatment instituted if an allergic-like reaction occurs or is suspected.
Influenza can be associated with a variety of neurologic and behavioral symptoms that can include events such as hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease.
There have been postmarketing reports (mostly from Japan) of delirium and abnormal behavior leading to injury, and in some cases resulting in fatal outcomes, in patients with influenza who were receiving TAMIFLU. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made but they appear to be uncommon based on TAMIFLU usage data. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of TAMIFLU to these events has not been established. Closely monitor patients with influenza for signs of abnormal behavior. If neuropsychiatric symptoms occur, evaluate the risks and benefits of continuing treatment for each patient.
Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. TAMIFLU has not been shown to prevent such complications.
Efficacy of TAMIFLU in the treatment of influenza in patients with chronic cardiac disease and/or respiratory disease has not been established. No difference in the incidence of complications was observed between the treatment and placebo groups in this population. No information is available regarding treatment of influenza in patients with any medical condition sufficiently severe or unstable to be considered at imminent risk of requiring hospitalization.
Efficacy of TAMIFLU for treatment or prophylaxis of influenza has not been established in immunocompromised patients.
Safety and efficacy of TAMIFLU for treatment of influenza in pediatric patients less than 2 weeks of age have not been established. Safety and efficacy of TAMIFLU for prophylaxis of influenza have not been established for pediatric patients less than 1 year of age.
TAMIFLU ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Serious skin and hypersensitivity reactions [see Warnings and Precautions (5.1)]
- Neuropsychiatric events [see Warnings and Precautions (5.2)]
The most common adverse reactions are nausea and vomiting.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Treatment Studies in Adult and Adolescent Subjects (13 years of age and older)
A total of 1171 subjects who participated in adult controlled clinical trials for the treatment of influenza were treated with TAMIFLU. The most frequently reported adverse events in these studies were nausea and vomiting. These events were generally of mild to moderate severity and usually occurred on the first 2 days of administration. Less than 1% of subjects discontinued prematurely from clinical trials due to nausea and vomiting.
Adverse events that occurred with an incidence of 1% or greater in 1440 subjects taking placebo or TAMIFLU 75 mg twice daily in adult treatment studies are shown in Table 4. This summary includes 945 healthy young adults and 495 "at risk" subjects (elderly patients and patients with chronic cardiac or respiratory disease). Those events reported numerically more frequently in subjects taking TAMIFLU compared with placebo were nausea, vomiting, bronchitis, insomnia, and vertigo.
Prophylaxis Studies in Adult and Adolescent Subjects (13 years of age and older)
A total of 4187 subjects (adolescents, healthy adults, and elderly) participated in prophylaxis studies, of whom 1790 received the recommended dose of 75 mg once daily for up to 6 weeks. Adverse events were qualitatively very similar to those seen in the treatment studies, despite a longer duration of dosing (see Table 4). Events reported more frequently in subjects receiving TAMIFLU compared to subjects receiving placebo in prophylaxis studies, and more commonly than in treatment studies, were aches and pains, rhinorrhea, dyspepsia and upper respiratory tract infections. However, the difference in incidence between TAMIFLU and placebo for these events was less than 1%. There were no clinically relevant differences in the safety profile of the 942 elderly subjects who received TAMIFLU or placebo, compared with the younger population.
| Treatment | Prophylaxis | |||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event * | Placebo | TAMIFLU 75 mg twice daily |
Placebo/ No Prophylaxis† |
TAMIFLU 75 mg once daily |
||||
| N=716 | N=724 | N=1688 | N=1790 | |||||
| Nausea (without vomiting) | 40 | (6%) | 72 | (10%) | 56 | (3%) | 129 | (7%) |
| Vomiting | 21 | (3%) | 68 | (9%) | 16 | (1%) | 39 | (2%) |
| Diarrhea | 70 | (10%) | 48 | (7%) | 40 | (2%) | 50 | (3%) |
| Bronchitis | 15 | (2%) | 17 | (2%) | 22 | (1%) | 15 | (1%) |
| Abdominal pain | 16 | (2%) | 16 | (2%) | 25 | (1%) | 37 | (2%) |
| Dizziness | 25 | (3%) | 15 | (2%) | 21 | (1%) | 24 | (1%) |
| Headache | 14 | (2%) | 13 | (2%) | 306 | (18%) | 326 | (18%) |
| Cough | 12 | (2%) | 9 | (1%) | 119 | (7%) | 94 | (5%) |
| Insomnia | 6 | (1%) | 8 | (1%) | 15 | (1%) | 22 | (1%) |
| Vertigo | 4 | (1%) | 7 | (1%) | 4 | (<1%) | 4 | (<1%) |
| Fatigue | 7 | (1%) | 7 | (1%) | 163 | (10%) | 139 | (8%) |


Additional adverse events occurring in less than 1% of patients receiving TAMIFLU for treatment included unstable angina, anemia, pseudomembranous colitis, humerus fracture, pneumonia, pyrexia, and peritonsillar abscess.
Treatment Studies in Pediatric Subjects (1 to 12 years of age)
A total of 1032 pediatric subjects aged 1 to 12 years (including 698 otherwise healthy pediatric subjects aged 1 to 12 years and 334 asthmatic pediatric subjects aged 6 to 12 years) participated in controlled clinical trials of TAMIFLU given for the treatment of influenza. A total of 515 pediatric subjects received treatment with TAMIFLU for oral suspension.
Adverse events occurring in 1% or greater of pediatric subjects receiving TAMIFLU treatment are listed in Table 5. The most frequently reported adverse event was vomiting. Other events reported more frequently by pediatric subjects treated with TAMIFLU included abdominal pain, epistaxis, ear disorder, and conjunctivitis. These events generally occurred once and resolved despite continued dosing resulting in discontinuation of drug in 8 out of 515 (2%) cases.
The adverse event profile in adolescents is similar to that described for adult subjects and pediatric subjects aged 1 to 12 years.
Prophylaxis Studies in Pediatric Subjects (1 to 12 years of age)
Pediatric subjects aged 1 to 12 years participated in a postexposure prophylaxis study in households, both as index cases (n=134) and as contacts (n=222). Gastrointestinal events were the most frequent, particularly vomiting. In a separate 6-week, uncontrolled, pediatric seasonal prophylaxis study (n=49), the adverse events noted were consistent with those previously observed (see Table 5).
| Treatment Trials* | Household Prophylaxis Trial† | |||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event‡ | Placebo N=517 |
TAMIFLU 2 mg/kg twice daily N=515 |
No Prophylaxis N=87 |
Prophylaxis with TAMIFLU once daily§
N=99 |
||||
| Vomiting | 48 | (9%) | 77 | (15%) | 2 | (2%) | 10 | (10%) |
| Diarrhea | 55 | (11%) | 49 | (10%) | - | 1 | (1%) | |
| Otitis media | 58 | (11%) | 45 | (9%) | 2 | (2%) | 2 | (2%) |
| Abdominal pain | 20 | (4%) | 24 | (5%) | - | 3 | (3%) | |
| Asthma (including aggravated) | 19 | (4%) | 18 | (3%) | 1 | (1%) | 1 | (1%) |
| Nausea | 22 | (4%) | 17 | (3%) | 1 | (1%) | 4 | (4%) |
| Epistaxis | 13 | (3%) | 16 | (3%) | - | 1 | (1%) | |
| Pneumonia | 17 | (3%) | 10 | (2%) | 2 | (2%) | - | |
| Ear disorder | 6 | (1%) | 9 | (2%) | - | - | ||
| Sinusitis | 13 | (3%) | 9 | (2%) | - | - | ||
| Bronchitis | 11 | (2%) | 8 | (2%) | 2 | (2%) | - | |
| Conjunctivitis | 2 | (<1%) | 5 | (1%) | - | - | ||
| Dermatitis | 10 | (2%) | 5 | (1%) | - | - | ||
| Lymphadenopathy | 8 | (2%) | 5 | (1%) | - | - | ||
| Tympanic membrane disorder | 6 | (1%) | 5 | (1%) | - | - | ||




Treatment Studies in Pediatric Subjects (2 weeks to less than 1 year of age)
Assessment of adverse reactions is based on two open label studies that included safety data on 135 influenza-infected subjects 2 weeks to less than 1 year of age (including premature infants at least 36 weeks post conceptional age) exposed to TAMIFLU at doses ranging from 2 to 3.5 mg/kg twice daily for 5 days. The safety profile was similar across the age range studied, with vomiting, diarrhea and diaper rash being the most frequently reported adverse reactions. The safety profile observed in subjects 2 weeks to less than 1 year of age was consistent with the established safety profile of adults and pediatric subjects older than 1 year of age.
Prophylaxis Study in Immunocompromised Subjects
In a 12-week seasonal prophylaxis study in 475 immunocompromised subjects, including 18 pediatric subjects 1 to 12 years of age, the safety profile in the 238 subjects receiving TAMIFLU was consistent with that previously observed in other TAMIFLU prophylaxis clinical trials.
The following adverse reactions have been identified during postapproval use of TAMIFLU. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to TAMIFLU exposure.
Body as a Whole: Swelling of the face or tongue, allergy, anaphylactic/anaphylactoid reactions, hypothermia
Dermatologic: Rash, dermatitis, urticaria, eczema, toxic epidermal necrolysis, Stevens-Johnson Syndrome, erythema multiforme [see Warnings and Precautions (5.1)]
Digestive: Hepatitis, liver function tests abnormal
Cardiac: Arrhythmia
Gastrointestinal disorders: Gastrointestinal bleeding, hemorrhagic colitis
Neurologic: Seizure
Metabolic: Aggravation of diabetes
Psychiatric: Abnormal behavior, delirium, including symptoms such as hallucinations, agitation, anxiety, altered level of consciousness, confusion, nightmares, delusions [see Warnings and Precautions (5.2)]
DRUG INTERACTIONS
Live attenuated influenza vaccine, intranasal (7):
- Do not administer until 48 hours following cessation of TAMIFLU.
- Do not administer TAMIFLU until 2 weeks following administration of the live attenuated influenza vaccine, unless medically indicated.
Influenza Vaccines
The concurrent use of TAMIFLU with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of the potential for interference between these products, LAIV should not be administered within 2 weeks before or 48 hours after administration of TAMIFLU, unless medically indicated. The concern about possible interference arises from the potential for antiviral drugs to inhibit replication of live vaccine virus. Trivalent inactivated influenza vaccine can be administered at any time relative to use of TAMIFLU.
Overall Drug Interaction Profile for Oseltamivir
Information derived from pharmacology and pharmacokinetic studies of oseltamivir suggests that clinically significant drug interactions are unlikely.
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases, located predominantly in the liver. Drug interactions involving competition for esterases have not been extensively reported in literature. Low protein binding of oseltamivir and oseltamivir carboxylate suggests that the probability of drug displacement interactions is low.
In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases.
Clinically important drug interactions involving competition for renal tubular secretion are unlikely due to the known safety margin for most of these drugs, the elimination characteristics of oseltamivir carboxylate (glomerular filtration and anionic tubular secretion) and the excretion capacity of these pathways. Coadministration of probenecid results in an approximate two-fold increase in exposure to oseltamivir carboxylate due to a decrease in active anionic tubular secretion in the kidney. However, due to the safety margin of oseltamivir carboxylate, no dose adjustments are required when coadministering with probenecid.
No pharmacokinetic interactions have been observed when coadministering oseltamivir with amoxicillin, acetaminophen, aspirin, cimetidine, antacids (magnesium and aluminum hydroxides and calcium carbonates), or warfarin.
USE IN SPECIFIC POPULATIONS
- Pregnancy: No data in pregnant women. Use only if clearly needed. (8.1)
- Nursing mothers: Caution should be exercised when administered to a nursing woman. (8.3).
Pregnancy Category C
There are insufficient human data upon which to base an evaluation of risk of TAMIFLU to the pregnant woman or developing fetus. Studies for effects on embryo-fetal development were conducted in rats (50, 250, and 1500 mg/kg/day) and rabbits (50, 150, and 500 mg/kg/day) by the oral route. Relative exposures at these doses were, respectively, 2, 13, and 100 times human exposure in the rat and 4, 8, and 50 times human exposure in the rabbit. Pharmacokinetic studies indicated that fetal exposure was seen in both species. In the rat study, minimal maternal toxicity was reported in the 1500 mg/kg/day group. In the rabbit study, slight and marked maternal toxicities were observed, respectively, in the 150 and 500 mg/kg/day groups. There was a dose-dependent increase in the incidence rates of a variety of minor skeletal abnormalities and variants in the exposed offspring in these studies. However, the individual incidence rate of each skeletal abnormality or variant remained within the background rates of occurrence in the species studied.
Because animal reproductive studies may not be predictive of human response and there are no adequate and well-controlled studies in pregnant women, TAMIFLU should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In lactating rats, oseltamivir and oseltamivir carboxylate are excreted in the milk. It is not known whether oseltamivir or oseltamivir carboxylate is excreted in human milk. TAMIFLU should, therefore, be used only if the potential benefit for the lactating mother justifies the potential risk to the breast-fed infant.
Safety and efficacy of TAMIFLU for treatment of influenza in pediatric patients less than 2 weeks of age have not been established. Safety and efficacy of TAMIFLU for prophylaxis of influenza have not been established for pediatric patients less than 1 year of age.
Of the total number of subjects in clinical studies of TAMIFLU for the treatment of influenza, 19% were 65 and over, while 7% were 75 and over. Of the total number of subjects in clinical studies of TAMIFLU for the prophylaxis of influenza, 25% were 65 and over, while 18% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
The safety of TAMIFLU in geriatric subjects has been established in clinical studies that enrolled 741 subjects (374 received placebo and 362 received TAMIFLU). Some seasonal variability was noted in the clinical efficacy outcomes [see Clinical Studies (14.1)].
Safety and efficacy have been demonstrated in elderly residents of nursing homes who took TAMIFLU for up to 42 days for the prevention of influenza. Many of these individuals had cardiac and/or respiratory disease, and most had received vaccine that season [see Clinical Studies (14.2)].
Dose adjustment is recommended for patients with a serum creatinine clearance between 10 and 30 mL/min [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. No recommended dosing regimens are available for patients with end-stage renal disease undergoing routine hemodialysis or continuous peritoneal dialysis treatment.
No dosage adjustment is required in patients with mild to moderate hepatic impairment. The safety and pharmacokinetics in patients with severe hepatic impairment have not been evaluated [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
OVERDOSAGE
Reports of overdoses with TAMIFLU have been received from clinical trials and during postmarketing experience. In the majority of cases reporting overdose, no adverse events were reported. Adverse events reported following overdose were similar in nature to those observed with therapeutic doses of TAMIFLU [see Adverse Reactions (6)].
TAMIFLU DESCRIPTION
TAMIFLU (oseltamivir phosphate) is available as capsules containing 30 mg, 45 mg, or 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 6 mg/mL oseltamivir base. In addition to the active ingredient, each capsule contains pregelatinized starch, talc, povidone K30, croscarmellose sodium, and sodium stearyl fumarate. The 30 mg capsule shell contains gelatin, titanium dioxide, yellow iron oxide, and red iron oxide. The 45 mg capsule shell contains gelatin, titanium dioxide, and black iron oxide. The 75 mg capsule shell contains gelatin, titanium dioxide, yellow iron oxide, black iron oxide, and red iron oxide. Each capsule is printed with blue ink, which includes FD&C Blue No. 2 as the colorant. In addition to the active ingredient, the powder for oral suspension contains sorbitol, monosodium citrate, xanthan gum, titanium dioxide, tutti-frutti flavoring, sodium benzoate, and saccharin sodium.
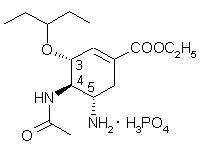
Oseltamivir phosphate is a white crystalline solid with the chemical name (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C16H28N2O4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The structural formula is as follows:
CLINICAL PHARMACOLOGY
Oseltamivir is an antiviral drug [see Microbiology(12.4)].
Absorption and Bioavailability
Oseltamivir is readily absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted predominantly by hepatic esterases to oseltamivir carboxylate. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate. Exposure to oseltamivir is less than 5% of the total exposure after oral dosing (see Table 6).
| Parameter | Oseltamivir | Oseltamivir Carboxylate |
|---|---|---|
| Cmax (ng/mL) | 65 (26) | 348 (18) |
| AUC0-12h (ng∙h/mL) | 112 (25) | 2719 (20) |
Plasma concentrations of oseltamivir carboxylate are proportional to doses up to 500 mg given twice daily.
Coadministration with food has no significant effect on the peak plasma concentration (551 ng/mL under fasted conditions and 441 ng/mL under fed conditions) and the area under the plasma concentration time curve (6218 ng∙h/mL under fasted conditions and 6069 ng∙h/mL under fed conditions) of oseltamivir carboxylate.
Distribution
The volume of distribution (Vss) of oseltamivir carboxylate, following intravenous administration in 24 subjects, ranged between 23 and 26 liters.
The binding of oseltamivir carboxylate to human plasma protein is low (3%). The binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions.
Metabolism
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases located predominantly in the liver. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms.
Elimination
Absorbed oseltamivir is primarily (>90%) eliminated by conversion to oseltamivir carboxylate. Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration. Oseltamivir carboxylate is not further metabolized and is eliminated in the urine. Plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration. Oseltamivir carboxylate is eliminated entirely (>99%) by renal excretion. Renal clearance (18.8 L/h) exceeds glomerular filtration rate (7.5 L/h), indicating that tubular secretion occurs in addition to glomerular filtration. Less than 20% of an oral radiolabeled dose is eliminated in feces.
Special Populations
Renal Impairment
Administration of 100 mg of oseltamivir phosphate twice daily for 5 days to subjects with various degrees of renal impairment showed that exposure to oseltamivir carboxylate is inversely proportional to declining renal function. Oseltamivir carboxylate exposures in subjects with normal and impaired renal function administered various dose regimens of oseltamivir are described in Table 7.
| Parameter | Normal Renal Function | Impaired Renal Function | ||||||
|---|---|---|---|---|---|---|---|---|
| 75 mg once daily | 75 mg twice daily | 150 mg twice daily | Creatinine Clearance <10 mL/min |
Creatinine Clearance >10 and <30 mL/min |
||||
| CAPD | Hemodialysis | 75 mg daily | 75 mg alternate days | 30 mg daily | ||||
| 30 mg weekly | 30 mg alternate HD cycle | |||||||
| Cmax | 259* | 348 |
705 |
766 | 850 | 1638 | 1175 | 655 |
| Cmin | 39 |
138 |
288 |
62 | 48 | 864 | 209 | 346 |
| AUC48 † | 7476 |
10876 |
21864 |
17381 | 12429 | 62636 | 21999 | 25054 |


Hepatic Impairment
In clinical studies oseltamivir carboxylate exposure was not altered in subjects with mild or moderate hepatic impairment [see Dosage and Administration (2.5) and Use in Specific Populations (8.7)].
Pediatric Subjects (1 to 12 years of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in a single-dose pharmacokinetic study in pediatric subjects aged 5 to 16 years (n=18) and in a small number of pediatric subjects aged 3 to 12 years (n=5) enrolled in a clinical trial. Younger pediatric subjects cleared both the prodrug and the active metabolite faster than adult subjects resulting in a lower exposure for a given mg/kg dose. For oseltamivir carboxylate, apparent total clearance decreases linearly with increasing age (up to 12 years). The pharmacokinetics of oseltamivir in pediatric subjects over 12 years of age are similar to those in adult subjects.
Pediatric Subjects (2 weeks to less than 1 year of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in two open-label studies of pediatric subjects less than one year of age (n=122) infected with influenza. Apparent clearance of the active metabolite decreases with decreasing age in subjects less than 1 year of age; however the oseltamivir and oseltamivir carboxylate exposure following a 3 mg/kg dose in subjects under 1 year of age is expected to be within the observed exposures in adults and adolescents receiving 75 mg twice daily and 150 mg twice daily.
Geriatric Patients
Exposure to oseltamivir carboxylate at steady-state was 25% to 35% higher in geriatric subjects (age range 65 to 78 years) compared to young adults given comparable doses of oseltamivir. Half-lives observed in the geriatric subjects were similar to those seen in young adults. Based on drug exposure and tolerability, dose adjustments are not required for geriatric patients for either treatment or prophylaxis [see Dosage and Administration (2.6)].
Mechanism of Action
Oseltamivir phosphate is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form, oseltamivir carboxylate. Oseltamivir carboxylate is an inhibitor of influenza virus neuraminidase affecting release of viral particles.
Antiviral Activity
The antiviral activity and neuraminidase inhibitory activity of oseltamivir carboxylate against laboratory strains and clinical isolates of influenza virus was determined in cell culture and biochemical assays. The concentrations of oseltamivir carboxylate required for inhibition of influenza virus in cell culture were highly variable depending on the assay method used and the virus tested. The 50% and 90% effective concentrations (EC50 and EC90) were in the range of 0.0008 µM to >35 µM and 0.004 µM to >100 µM, respectively (1 µM=0.284 µg/mL). The median IC50 values of oseltamivir against influenza A/H1N1, influenza A/H3N2, and influenza B clinical isolates were 2.5 nM (range 0.93-4.16 nM, N=74), 0.96 nM (range 0.13-7.95 nM, N=774), and 60 nM (20-285 nM, N=256), respectively, in a neuraminidase assay with a fluorescently labeled MUNANA substrate. The relationship between the antiviral activity in cell culture, inhibitory activity in the neuraminidase assay, and the inhibition of influenza virus replication in humans has not been established.
Resistance
Influenza A virus isolates with reduced susceptibility to oseltamivir carboxylate have been recovered by serial passage of virus in cell culture in the presence of increasing concentrations of oseltamivir carboxylate, from clinical isolates collected during treatment with oseltamivir, and from viral isolates sampled during community surveillance studies. Reduced susceptibility of influenza virus to inhibition by oseltamivir carboxylate may be conferred by amino acid substitutions in the viral neuraminidase and/or hemagglutinin proteins. Changes in the viral neuraminidase that have been associated with reduced susceptibility to oseltamivir carboxylate are summarized in Table 8. Hemagglutinin substitutions associated with oseltamivir resistance include A28T and R124M in influenza A H3N2 and H154Q in H1N9, a reassortant human/avian virus.
| Amino Acid Substitution | Influenza Type/ Sub-type |
Source |
|---|---|---|
| Catalytic Residues | ||
| R292K | A N2 | Roche clinical trials, publication, surveillance* |
| Framework Residues | ||
| H275Y | A N1 | Roche clinical trials, publication, surveillance |
| N294S | A N1, N2 | Publications |
| E119V | A N2 | Roche clinical trials, publication, surveillance |
| SASG245-248 deletion | A N2 | Roche clinical trial |
| I222V | A N2 | Publication |
| I222T | B | Publication |
| D198N | B | Publication, surveillance |
| D198E | B | Surveillance |
| R371K | B | Surveillance |
| G402S | B | Publication |

Selection of influenza A viruses resistant to oseltamivir can occur at higher frequencies in children. The incidence of oseltamivir treatment-associated resistance in pediatric treatment studies has been detected at rates of 27% to 37% and 3% to 18% (3/11 to 7/19 and 1/34 to 9/50 post-treatment isolates, respectively) for influenza A/H1N1 and influenza A/H3N2, respectively. The frequency of resistance selection to oseltamivir and the prevalence of such resistant virus vary seasonally and geographically.
Circulating seasonal influenza strains expressing neuraminidase resistance-associated substitutions have been observed in individuals who have not received oseltamivir treatment. The oseltamivir resistance-associated substitution H275Y was found in >99% of US circulating 2008 H1N1 influenza isolates. The 2009 H1N1 influenza ("swine flu") was almost uniformly susceptible to oseltamivir. Prescribers should consider available information from the CDC on influenza drug susceptibility patterns and treatment effects when deciding whether to use TAMIFLU.
Cross-resistance
Cross-resistance between oseltamivir and zanamivir has been observed in neuraminidase biochemical assays. The H275Y (N1 numbering) or N294S (N2 numbering) oseltamivir resistance-associated substitutions observed in the N1 neuraminidase subtype, and the E119V or N294S oseltamivir resistance-associated substitutions observed in the N2 subtype (N2 numbering), are associated with reduced susceptibility to oseltamivir but not zanamivir. The Q136K and K150T zanamivir resistance-associated substitutions observed in N1 neuraminidase, or the S250G zanamivir resistance-associated substitutions observed in influenza B, confer reduced susceptibility to zanamivir but not oseltamivir. The R292K oseltamivir resistance-associated substitution observed in N2, and the I222T, D198E/N, R371K, or G402S oseltamivir resistance-associated substitutions observed in influenza B neuraminidase, confer reduced susceptibility to both oseltamivir and zanamivir. In general, amino acid substitutions at neuraminidase catalytic residues confer cross-resistance to other neuraminidase inhibitors while substitutions at framework residues may or may not confer cross-resistance.
No single amino acid substitution has been identified that could confer cross-resistance between the neuraminidase inhibitor class (oseltamivir, zanamivir) and the M2 ion channel inhibitor class (amantadine, rimantadine). However, a virus may carry a neuraminidase inhibitor associated substitution in neuraminidase and an M2 ion channel inhibitor associated substitution in M2 and may therefore be resistant to both classes of inhibitors. The clinical relevance of phenotypic cross-resistance evaluations has not been established.
Immune Response
No influenza vaccine/oseltamivir interaction study has been conducted. In studies of naturally acquired and experimental influenza, treatment with TAMIFLU did not impair normal humoral antibody response to infection.
NONCLINICAL TOXICOLOGY
In 2-year carcinogenicity studies in mice and rats given daily oral doses of the prodrug oseltamivir phosphate up to 400 mg/kg and 500 mg/kg, respectively, the prodrug and the active form oseltamivir carboxylate induced no statistically significant increases in tumors over controls. The mean maximum daily exposures to the prodrug in mice and rats were approximately 130- and 320-fold, respectively, greater than those in humans at the proposed clinical dose based on AUC comparisons. The respective safety margins of the exposures to the active oseltamivir carboxylate were 15- and 50-fold.
Oseltamivir was found to be non-mutagenic in the Ames test and the human lymphocyte chromosome assay with and without enzymatic activation and negative in the mouse micronucleus test. It was found to be positive in a Syrian Hamster Embryo (SHE) cell transformation test. Oseltamivir carboxylate was non-mutagenic in the Ames test and the L5178Y mouse lymphoma assay with and without enzymatic activation and negative in the SHE cell transformation test.
In a fertility and early embryonic development study in rats, doses of oseltamivir at 50, 250, and 1500 mg/kg/day were administered to females for 2 weeks before mating, during mating and until day 6 of pregnancy. Males were dosed for 4 weeks before mating, during mating, and for 2 weeks after mating. There were no effects on fertility, mating performance or early embryonic development at any dose level. The highest dose was approximately 100 times the human systemic exposure (AUC0-24h) of oseltamivir carboxylate.
Single, oral administration of ≥657 mg/kg oseltamivir resulted in toxicity, including death, in juvenile 7 day old rats, but had no effect on adult rats. No toxicity was observed after repeated administration of up to 500 mg/kg oseltamivir to developing juvenile rats 7 to 21 days old. This 500 mg/kg dose was approximately 280 and 14 times the human systemic exposure (AUC0-24h) of oseltamivir and oseltamivir carboxylate, respectively. Clinical relevance of the juvenile rat study finding for young infants is unknown.
CLINICAL STUDIES
Adult and Adolescents Subjects (13 years of age and older)
Two placebo-controlled double-blind clinical trials were conducted: one in the U.S. and one outside the U.S. Subjects were eligible for these trials if they had fever >100°F, accompanied by at least one respiratory symptom (cough, nasal symptoms, or sore throat) and at least one systemic symptom (myalgia, chills/sweats, malaise, fatigue, or headache) and influenza virus was known to be circulating in the community. In addition, all subjects enrolled in the trials were allowed to take fever-reducing medications.
Of 1355 subjects enrolled in these two trials, 849 (63%) subjects were influenza-infected (age range 18 to 65 years; median age 34 years; 52% male; 90% Caucasian; 31% smokers). Of the 849 influenza-infected subjects, 95% were infected with influenza A, 3% with influenza B, and 2% with influenza of unknown type.
TAMIFLU was started within 40 hours of onset of symptoms. Subjects participating in the trials were required to self-assess the influenza-associated symptoms as "none," "mild," "moderate," or "severe." Time to improvement was calculated from the time of treatment initiation to the time when all symptoms (nasal congestion, sore throat, cough, aches, fatigue, headaches, and chills/sweats) were assessed as "none" or "mild." In both studies, at the recommended dose of TAMIFLU 75 mg twice daily for 5 days, there was a 1.3 day reduction in the median time to improvement in influenza-infected subjects receiving TAMIFLU compared to subjects receiving placebo. Subgroup analyses of these studies by gender showed no differences in the treatment effect of TAMIFLU in men and women.
In the treatment of influenza, no increased efficacy was demonstrated in subjects receiving treatment of 150 mg TAMIFLU twice daily for 5 days.
Geriatric Subjects
Three double-blind placebo-controlled treatment trials were conducted in subjects ≥65 years of age in three consecutive seasons. The enrollment criteria were similar to that of adult trials with the exception of fever being defined as >97.5°F. Of 741 subjects enrolled, 476 (65%) subjects were influenza-infected. Of the 476 influenza-infected subjects, 95% were infected with influenza type A and 5% with influenza type B.
In the pooled analysis, at the recommended dose of TAMIFLU 75 mg twice daily for 5 days, there was a 1-day reduction in the median time to improvement in influenza-infected subjects receiving TAMIFLU compared to those receiving placebo (p=NS). However, the magnitude of treatment effect varied between studies.
Pediatric Subjects (1 to 12 years of age)
One double-blind placebo-controlled treatment trial was conducted in pediatric subjects aged 1 to 12 years (median age 5 years), who had fever (>100°F) plus one respiratory symptom (cough or coryza) when influenza virus was known to be circulating in the community. Of 698 subjects enrolled in this trial, 452 (65%) were influenza-infected (50% male; 68% Caucasian). Of the 452 influenza-infected subjects, 67% were infected with influenza A and 33% with influenza B.
The primary endpoint in this study was the time to freedom from illness, a composite endpoint that required 4 individual conditions to be met. These were: alleviation of cough, alleviation of coryza, resolution of fever, and parental opinion of a return to normal health and activity. TAMIFLU treatment of 2 mg/kg twice daily, started within 48 hours of onset of symptoms, significantly reduced the total composite time to freedom from illness by 1.5 days compared to placebo. Subgroup analyses of this study by gender showed no differences in the treatment effect of TAMIFLU in male and female pediatric subjects.
Pediatric Subjects (2 weeks to less than 1 year of age)
Two open label trials evaluated the safety and pharmacokinetics of oseltamivir and oseltamivir carboxylate in influenza infected pediatric subjects 2 weeks to less than 1 year of age (including premature infants at least 36 weeks post conceptional age). Subjects received TAMIFLU at doses ranging from 2 to 3.5 mg/kg twice daily for 5 days depending on subject age. These clinical trials were not designed to evaluate clinical efficacy or virologic response.
Of the 136 subjects under the age of 1 year enrolled and dosed in the trials, the majority of the subjects were male (55%), white (79%), non-Hispanic (74%), full term (76%) and infected with influenza A (80%). Pharmacokinetic data indicated that a dose of 3 mg/kg twice daily in pediatric subjects 2 weeks to less than 1 year of age provided TAMIFLU concentrations similar to or higher than those observed in older pediatric subjects and adults receiving the approved dose and, by extrapolation, is expected to provide similar efficacy. The trials provided adequate safety data to support this dose selection [see Adverse Reactions (6.1) ].
Adult and Adolescent Subjects (13 years of age and older)
The efficacy of TAMIFLU in preventing naturally occurring influenza illness has been demonstrated in three seasonal prophylaxis studies and a postexposure prophylaxis study in households. The primary efficacy parameter for all these studies was the incidence of laboratory-confirmed clinical influenza. Laboratory-confirmed clinical influenza was defined as oral temperature ≥99.0°F/37.2°C plus at least one respiratory symptom (cough, sore throat, nasal congestion) and at least one constitutional symptom (aches and pain, fatigue, headache, chills/sweats), all recorded within 24 hours, plus either a positive virus isolation or a four-fold increase in virus antibody titers from baseline.
In a pooled analysis of two seasonal prophylaxis studies in healthy unvaccinated adults (aged 13 to 65 years), TAMIFLU 75 mg once daily taken for 42 days during a community outbreak reduced the incidence of laboratory-confirmed clinical influenza from 5% (25/519) for the placebo group to 1% (6/520) for the TAMIFLU group.
In a seasonal prophylaxis study in elderly residents of skilled nursing homes, TAMIFLU 75 mg once daily taken for 42 days reduced the incidence of laboratory-confirmed clinical influenza from 4% (12/272) for the placebo group to <1% (1/276) for the TAMIFLU group. About 80% of this elderly population were vaccinated, 14% of subjects had chronic airway obstructive disorders, and 43% had cardiac disorders.
In a study of postexposure prophylaxis in household contacts (aged ≥13 years) of an index case, TAMIFLU 75 mg once daily administered within 2 days of onset of symptoms in the index case and continued for 7 days reduced the incidence of laboratory-confirmed clinical influenza from 12% (24/200) in the placebo group to 1% (2/205) for the TAMIFLU group. Index cases did not receive TAMIFLU in the study.
Pediatric Subjects (1 to 12 years of age)
The efficacy of TAMIFLU in preventing naturally occurring influenza illness has been demonstrated in a randomized, open-label, postexposure prophylaxis study in households that included pediatric subjects aged 1 to 12 years, both as index cases and as family contacts. All index cases in this study received treatment. The primary efficacy parameter for this study was the incidence of laboratory-confirmed clinical influenza in the household. Laboratory-confirmed clinical influenza was defined as oral temperature ≥100°F/37.8°C plus cough and/or coryza recorded within 48 hours, plus either a positive virus isolation or a four-fold or greater increase in virus antibody titers from baseline or at illness visits. Among household contacts 1 to 12 years of age not already shedding virus at baseline, TAMIFLU for oral suspension 30 mg to 60 mg taken once daily for 10 days reduced the incidence of laboratory-confirmed clinical influenza from 17% (18/106) in the group not receiving prophylaxis to 3% (3/95) in the group receiving prophylaxis.
Immunocompromised Subjects
A double-blind, placebo-controlled study was conducted for seasonal prophylaxis of influenza in 475 immunocompromised subjects (including 18 pediatric subjects 1 to 12 years of age) who had received solid organ (n=388; liver, kidney, liver and kidney) or hematopoietic stem cell transplants (n=87). Median time since transplant for solid organ transplant recipients was 1105 days for the placebo group and 1379 days for the oseltamivir group. Median time since transplant for hematopoietic stem cell transplant recipients was 424 days for the placebo group and 367 days for the oseltamivir group. Approximately 40% of subjects received influenza vaccine prior to entering the study. The primary efficacy endpoint for this study was the incidence of confirmed, clinical influenza, defined as oral temperature >99.0°F/37.2°C plus cough and/or coryza, all recorded within 24 hours, plus either a positive virus culture or a four-fold increase in virus antibody titers from baseline. The incidence of confirmed clinical influenza was 3% (7/238) in the group not receiving TAMIFLU compared with 2% (5/237) in the group receiving TAMIFLU; this difference was not statistically significant. A secondary analysis was performed using the same clinical symptoms and RT-PCR for laboratory confirmation of influenza. Among subjects who were not already shedding virus at baseline, the incidence of RT-PCR-confirmed clinical influenza was 3% (7/231) in the group not receiving TAMIFLU and <1% (1/232) in the group receiving TAMIFLU.
HOW SUPPLIED
TAMIFLU Capsules
30-mg capsules (30 mg free base equivalent of the phosphate salt): light yellow hard gelatin capsules. "ROCHE" is printed in blue ink on the light yellow body and "30 mg" is printed in blue ink on the light yellow cap. Available in blister packages of 10 (NDC 0004-0802-85).
45-mg capsules (45 mg free base equivalent of the phosphate salt): grey hard gelatin capsules. "ROCHE" is printed in blue ink on the grey body and "45 mg" is printed in blue ink on the grey cap. Available in blister packages of 10 (NDC 0004-0801-85).
75-mg capsules (75 mg free base equivalent of the phosphate salt): grey/light yellow hard gelatin capsules. "ROCHE" is printed in blue ink on the grey body and "75 mg" is printed in blue ink on the light yellow cap. Available in blister packages of 10 (NDC 0004-0800-85).
Storage
Store the capsules at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
TAMIFLU for Oral Suspension
Supplied as a white powder blend in a glass bottle. After constitution, the powder blend produces a white tutti-frutti–flavored oral suspension. After constitution with 55 mL of water, each bottle delivers a usable volume of 60 mL of oral suspension equivalent to 360 mg oseltamivir base (6 mg/mL). Each bottle is supplied with a bottle adapter and a 10 mL oral dispenser (NDC 0004-0820-09).
Storage
Store dry powder at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Store constituted suspension under refrigeration for up to 17 days at 2° to 8°C (36° to 46°F). Do not freeze. Alternatively, store constituted suspension for up to 10 days at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
INFORMATION FOR PATIENTS
See FDA-approved Patient Labeling (Patient Information and Instructions for Use)
Patients and/or caregivers should be advised of the risk of severe allergic reactions (including anaphylaxis) or serious skin reactions and should stop TAMIFLU and seek immediate medical attention if an allergic-like reaction occurs or is suspected.
Patients and/or caregivers should be advised of the risk of neuropsychiatric events in patients with influenza and should contact their physician if they experience signs of abnormal behavior while receiving TAMIFLU. Their physician will determine if TAMIFLU treatment should be continued.
Instruct patients to begin treatment with TAMIFLU as soon as possible from the first appearance of flu symptoms. Similarly, prevention should begin as soon as possible after exposure, at the recommendation of a physician.
Instruct patients to take any missed doses as soon as they remember, except if it is near the next scheduled dose (within 2 hours), and then continue to take TAMIFLU at the usual times.
TAMIFLU is not a substitute for a flu vaccination. Patients should continue receiving an annual flu vaccination according to guidelines on immunization practices.
A bottle of TAMIFLU for oral suspension contains approximately 11 g sorbitol. One dose of 75 mg TAMIFLU for oral suspension delivers 2 g sorbitol. For patients with hereditary fructose intolerance, this is above the daily maximum limit of sorbitol and may cause dyspepsia and diarrhea.
SPL UNCLASSIFIED
Tamiflu® (oseltamivir phosphate)
Distributed by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
©2012 Genentech, Inc. All rights reserved
XXXXXXX
Licensor:
Gilead Sciences, Inc.
Foster City, California 94404
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION
TAMIFLU®
(TAM-ih-flew)
(oseltamivir phosphate) capsules, for oral use
TAMIFLU®
(TAM-ih-flew)
(oseltamivir phosphate)
for oral suspension
What is TAMIFLU?
TAMIFLU is a prescription medicine used to:
- treat the flu (influenza) in people 2 weeks of age and older who have had flu symptoms for no more than two days.
- prevent the flu in people who are 1 year of age and older.
It is not known if TAMIFLU is:
- effective in people who start treatment after 2 days of developing flu symptoms.
- effective for the treatment of the flu in people with long-time (chronic) heart problems or breathing problems.
- effective for the treatment or prevention of flu in people who have weakened immune systems (immunocompromised).
- safe and effective for the treatment of the flu in children less than 2 weeks of age.
- safe and effective in the prevention of the flu in children less than 1 year of age.
TAMIFLU does not treat or prevent illness that is caused by infections other than the influenza virus.
TAMIFLU does not prevent bacterial infections that may happen with the flu.
TAMIFLU does not take the place of receiving a flu vaccination. Talk to your healthcare provider about when you should receive an annual flu vaccination.
Who should not take TAMIFLU?
Do not take TAMIFLU if you are allergic to oseltamivir phosphate or any of the ingredients in TAMIFLU. See the end of this leaflet for a complete list of ingredients in TAMIFLU.
What should I tell my healthcare provider before taking TAMIFLU?
Before you take TAMIFLU, tell your healthcare provider if you:
- have problems swallowing TAMIFLU capsules.
- have kidney problems
- have a history of fructose (fruit sugar) intolerance. TAMIFLU contains sorbitol and may cause stomach upset and diarrhea in people who are fructose intolerant.
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if TAMIFLU will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if TAMIFLU passes into your breast milk. You and your healthcare provider should decide if you will take TAMIFLU while you are breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take TAMIFLU?
- Take TAMIFLU exactly as your healthcare provider tells you to.
- Take TAMIFLU with food or without food. There is less chance of stomach upset if you take TAMIFLU with food.
- If you miss a dose of TAMIFLU, take it as soon as you remember. If it is 2 hours or less before your next dose, do not take the missed dose. Take your next dose of TAMIFLU at your scheduled time. Do not take 2 doses at the same time.
- If TAMIFLU for oral suspension is not available or you cannot swallow TAMIFLU capsules, your healthcare provider may instruct you to open TAMIFLU capsules and mix the capsules contents with sweetened liquids such as chocolate syrup (regular or sugar-free), corn syrup, caramel topping, or light brown sugar (dissolved in water).
- If your healthcare provider has instructed you to take TAMIFLU oral suspension or open your TAMIFLU capsules, read the detailed Instructions for Use at the end of this leaflet.
What are the possible side effects of TAMIFLU?
TAMIFLU may cause serious side effects, including:
-
Serious skin and allergic reactions. TAMIFLU can cause serious skin and allergic reactions. Stop taking TAMIFLU and get medical help right away if you get any of the following symptoms:
- skin rash or hives
- your skin blisters and peels
- blisters or sores in your mouth
- itching
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing
- chest pain or tightness
- Change in behavior. People, especially children, who have the flu can develop nervous system problems and abnormal behavior that can lead to death. During treatment with TAMIFLU, tell your healthcare provider right away if you or your child have confusion, speech problems, shaky movements, seizures, or start hearing voices or seeing things that are not really there (hallucinations).
The most common side effects of TAMIFLU when used for treatment of the flu include nausea and vomiting.
The most common side effect of TAMIFLU when used for prevention of the flu include nausea, vomiting, diarrhea, and stomach (abdomen) pain.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of TAMIFLU.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TAMIFLU?
- Store TAMIFLU capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Store TAMIFLU for oral suspension in the refrigerator for up to 17 days between 36°F to 46°F (2°C to 8°C)
- Store TAMIFLU for oral suspension for up to 10 days at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away any unused TAMIFLU that is out of date or no longer needed.
Keep TAMIFLU and all medicines out of the reach of children.
General information about the safe and effective use of TAMIFLU.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TAMIFLU for a condition for which it was not prescribed. Do not give TAMIFLU to other people, even if they have the same symptoms you have. It mayharm them.
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about TAMIFLU that is written for health professionals.
For more information, go to www.tamiflu.com.
What are the ingredients in TAMIFLU?
Active ingredient: oseltamivir phosphate
Inactive ingredients:
TAMIFLU capsules: pregelatinized starch, talc, povidone K30, croscarmellose sodium, and sodium stearyl fumarate
30mg capsule shell: gelatin, titanium dioxide, yellow iron oxide, and red iron oxide
45mg capsules shell: gelatin, titanium dioxide, and black iron oxide
75mg capsules shell: gelatin, titanium dioxide, yellow iron oxide, black iron oxide, and red iron oxide
TAMIFLU for oral suspension: sorbitol, monosodium citrate, xanthan gum, titanium dioxide, tutti-frutti flavoring, sodium benzoate, saccharin sodium and water.
SPL PATIENT PACKAGE INSERT
Instructions For Use
TAMIFLU®
(TAM-ih-flew)
(oseltamivir phosphate)
capsules, for oral use
TAMIFLU®
(TAM-ih-flew)
(oseltamivir phosphate)
for oral suspension
How do I prepare a dose of TAMIFLU for oral suspension using the oral dispenser?
| Figure A. Oral Suspension | Figure B. Oral Dispenser |
|---|---|
|
|
Step 1. Shake the TAMIFLU for oral suspension bottle well before each use.
Step 2. Open the bottle by pushing downward on the child resistant bottle cap and twisting it in the direction of the arrow.
Step 3. Fully push down the plunger of the oral dispenser toward the tip before inserting into the bottle. Insert the tip of the oral dispenser into the opening of the bottle adapter.
Step 4. Turn the entire unit (bottle and oral dispenser) upside down.
Step 5. Gently pull back on the plunger of the oral dispenser until the bottom of the plunger is even with the line for the dose prescribed by your healthcare provider. (See Figure C ).
Figure C. Removing Oral Suspension
Step 6. Turn the entire unit right-side up and remove the oral dispenser slowly from the bottle by pulling straight up on the oral dispenser. Do not pull on plunger.
Step 7. Place the tip of the oral dispenser in the mouth. Press on the plunger to give the full contents of the oral dispenser into the mouth.
Step 8. Close the bottle with the child-resistant bottle cap after each use.
Step 9. Remove the plunger from the barrel, rinse both under running tap water and allow to air dry after each use.
How do I mix the contents of TAMIFLU capsules with sweetened liquids, if directed by my healthcare provider?
You will need:
- the prescribed dose of TAMIFLU capsules
- a small bowl
- sweetened liquid, such as chocolate syrup (regular or sugar-free), corn syrup, caramel topping, or light brown sugar (dissolved in water)
Step 1. Open the contents of the prescribed dose of TAMIFLU capsules into a small bowl.
Step 2. Add a small amount of the sweetened liquid to the capsule contents.
Step 3. Stir the mixture and give the entire dose of TAMIFLU.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
SPL UNCLASSIFIED
Tamiflu® (oseltamivir phosphate)
Distributed by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
©2012 Genentech, Inc. All rights reserved
Rev. December 2012
XXXXXXX
Licensor:
Gilead Sciences, Inc.
Foster City, California 94404
SPL UNCLASSIFIED
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Tamiflu
GENERIC: oseltamivir phosphate
DOSAGE: CAPSULE
ADMINSTRATION: ORAL
NDC: 52125-307-08
ACTIVE INGREDIENT(S):
- oseltamivir phosphate 75mg in 1
INACTIVE INGREDIENT(S):
- FD&C blue No. 2
- ferric oxide yellow
- ferrosoferric oxide
- ferric oxide red
- titanium dioxide
- gelatin
- povidone K30
- talc
- sodium stearyl fumarate
- croscarmellose sodium
- starch, corn
COLOR: gray
SHAPE: CAPSULE
SCORE: No score
SIZE: 18 mm
IMPRINT: ROCHE;75;mg
PACKAGING: 10 in 1 CARTON


Tamifluoseltamivir phosphate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||