System TLC Anti-dandruff
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Pyrithione Zinc 1%
Purpose
Anti-dandruff
Use
- Helps prevent recurrence of flaking and itching associated with dandruff
Warnings
- For external use only
- Not for use on larger areas of the body.
When using this product
- avoid contact with eyes. If this occurs, rinse thoroughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve with regular use of this product as directed.
Directions
For best results, use at least twice a week or as directed by a doctor. Wet hair thoroughly. Massage shampoo into your scalp for several minutes. Rinse and repeat. For maximum dandruff control, use everytime you shampoo. SHAKE WELL before use.
Inactive ingredients
Water, Ammonium Lauryl Sulfate, Ammonium Laureth Sulfate, Cocamidopropyl Betaine, Cocamide DEA, Lauramide DEA, Glycol Monosterate, Sodium Lauryl Sulfate, DMDM Hydantoin, Sodium Chloride, Fragrance, Citric Acid, FD&C Blue No.1.
Principal Display Panel
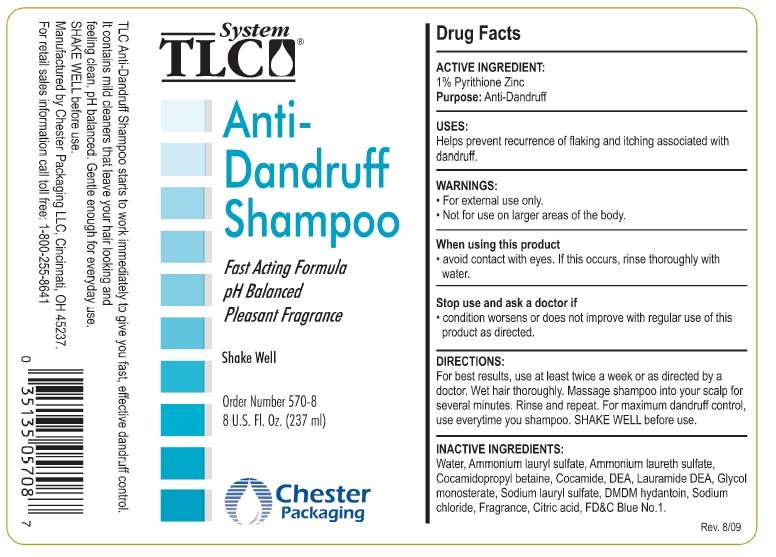
System TLC®
Anti-Dandruff Shampoo
Fast Acting Formula
pH Balanced
Pleasant Fragrance
Shake Well
Order Number 570-8
8 U. S. Fl. Oz. (237 ml)
Chester Packaging
System TLC Anti-dandruffPyrithione Zinc SHAMPOO
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||