Synthroid
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- SYNTHROID DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SYNTHROID CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SYNTHROID ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: Thyroid hormones, including SYNTHROID, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.SYNTHROID DESCRIPTION
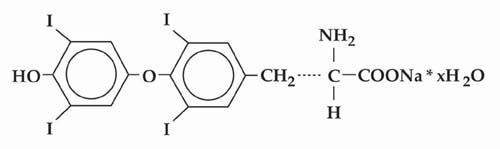
Inactive Ingredients
Strength (mcg) Color additive(s)
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
Pharmacokinetics
PRECAUTIONS - Drug InteractionsandDrug-Food Interactions).
PRECAUTIONS - Drug InteractionsandDrug-Laboratory Test Interactions). Thyroid hormones do not readily cross the placental barrier (seePRECAUTIONS - Pregnancy).
Table 1). The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately eighty-percent of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (rT3). T3 and rT3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Table 1. Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients
Hormone Ratio in Thyroglobulin Biologic Potency t1/2 (days) Protein Binding (%)2
INDICATIONS & USAGE
Hypothyroidism
Pituitary TSH Suppression
WARNINGSandPRECAUTIONS), including thyroid nodules (seeWARNINGSandPRECAUTIONS), subacute or chronic lymphocytic thyroiditis (Hashimoto's thyroiditis), multinodular goiter (seeWARNINGSandPRECAUTIONS) and, as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer.
SYNTHROID CONTRAINDICATIONS
PRECAUTIONS). SYNTHROID is contraindicated in patients with hypersensitivity to any of the inactive ingredients in SYNTHROID tablets (SeeDESCRIPTION - Inactive Ingredients).WARNINGS
Boxed WarningWARNING: Thyroid hormones, including SYNTHROID, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
CONTRAINDICATIONS). If the serum TSH level is not suppressed, SYNTHROID should be used with caution in conjunction with careful monitoring of thyroid function for evidence of hyperthyroidism and clinical monitoring for potential associated adverse cardiovascular signs and symptoms of hyperthyroidism.
PRECAUTIONS
GeneralDrug Interactions).
Effects on Bone Mineral Density
Patients with Underlying Cardiovascular Disease
WARNINGS,PRECAUTIONS - Geriatric Use, andDOSAGE AND ADMINISTRATION). If cardiac symptoms develop or worsen, the levothyroxine dose should be reduced or withheld for one week and then cautiously restarted at a lower dose. Overtreatment with levothyroxine sodium may have adverse cardiovascular effects such as an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias. Patients with coronary artery disease who are receiving levothyroxine therapy should be monitored closely during surgical procedures, since the possibility of precipitating cardiac arrhythmias may be greater in those treated with levothyroxine. Concomitant administration of levothyroxine and sympathomimetic agents to patients with coronary artery disease may precipitate coronary insufficiency.
Patients with Nontoxic Diffuse Goiter or Nodular Thyroid Disease
WARNINGS). If the serum TSH is already suppressed, levothyroxine sodium should not be administered (seeCONTRAINDICATIONS).
Associated Endocrine Disorders
PRECAUTIONS - Autoimmune polyglandular syndromefor adrenal insufficiency).
PRECAUTIONS - Drug Interactions).
Other Associated Medical Conditions
Information for Patients
Laboratory Tests
PRECAUTIONS - Drug InteractionsandDrug-Laboratory Test Interactions). Persistent clinical and laboratory evidence of hypothyroidism despite an apparent adequate replacement dose of SYNTHROID may be evidence of inadequate absorption, poor compliance, drug interactions, or decreased T4 potency of the drug product.
WARNINGS,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
(see PRECAUTIONS - Pediatric Use andDOSAGE AND ADMINISTRATION).
Drug Interactions
Table 2.
Table 2may not be comprehensive due to the introduction of new drugs that interact with the thyroidal axis or the discovery of previously unknown interactions. The prescriber should be aware of this fact and should consult appropriate reference sources (e.g., package inserts of newly approved drugs, medical literature) for additional information if a drug-drug interaction with levothyroxine is suspected.
Table 2. Drug-Thyroidal Axis Interactions
Drug or Drug Class Effect Drugs that may reduce TSH secretion
Drugs that alter thyroid hormone secretion Drugs that may decrease thyroid hormone secretion, which may result in hypothyroidism
Drugs that may increase thyroid hormone secretion, which may result in hyperthyroidism
Drugs that may decrease T4 absorption, which may result in hypothyroidism
Drugs that may increase serum TBG concentration Drugs that may decrease serum TBG concentration
Drugs that may cause protein-binding site displacement
Drugs that may alter T4 and T3 metabolism Drugs that may increase hepatic metabolism, which may result in hypothyroidism
Drugs that may decrease T4 5'-deiodinase activity
Miscellaneous
Table 2).
Table 2).
Drug-Food Interactions
Drug-Laboratory Test Interactions
Table 2). Familial hyper- or hypo-thyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Pregnancy
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION - Table 3). Dosing adjustments are based on an assessment of the individual patient's clinical and laboratory parameters (seePRECAUTIONS - Laboratory Tests).
PRECAUTIONS).
PRECAUTIONS - Laboratory TestsandDOSAGE AND ADMINISTRATION)
Geriatric Use
WARNINGS,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
SYNTHROID ADVERSE REACTIONS
PRECAUTIONSandOVERDOSAGE). They include the following:General
fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating;
Central nervous system
headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia;
Musculoskeletal
tremors, muscle weakness;
Cardiovascular
palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest;
Respiratory
dyspnea;
Gastrointestinal
diarrhea, vomiting, abdominal cramps and elevations in liver function tests;
Dermatologic
hair loss, flushing;
Endocrine
decreased bone mineral density;
Reproductive
Overdosage
PRECAUTIONSandADVERSE REACTIONS). In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures have occurred in a child ingesting 18 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Treatment of Overdosage
DOSAGE & ADMINISTRATION
General PrinciplesWARNINGSandPRECAUTIONS). Hence, the following recommendations serve only as dosing guidelines. Dosing must be individualized and adjustments made based on periodic assessment of the patient's clinical response and laboratory parameters (seePRECAUTIONS - Laboratory Tests).
PRECAUTIONS - Drug Interactions).
PRECAUTIONS).
Specific Patient Populations
WARNINGSandPRECAUTIONS - Laboratory Tests)
100-125 mcg/dayfor a 70 kg adult). Older patients may require less than 1 mcg/kg/day. Levothyroxine sodium doses greater than 200 mcg/day are seldom required. An inadequate response to daily doses300 mcg/day is rare and may indicate poor compliance, malabsorption, and/or drug interactions.
25-50 mcg/dayof levothyroxine sodium is recommended, with gradual increments in dose at 6-8 week intervals, as needed. The recommended starting dose of levothyroxine sodium in elderly patients with cardiac disease is 12.5-25 mcg/day, with gradual dose increments at 4-6 week intervals. The levothyroxine sodium dose is generally adjusted in 12.5-25 mcg increments until the patient with primary hypothyroidism is clinically euthyroid and the serum TSH has normalized.
12.5-25 mcg/daywith increases of 25 mcg/day every 2-4 weeks, accompanied by clinical and laboratory assessment, until the TSH level is normalized.
PRECAUTIONS - Laboratory Tests)
PRECAUTIONS - Pediatric Use). SYNTHROID may be administered to infants and children who cannot swallow intact tablets by crushing the tablet and suspending the freshly crushed tablet in a small amount (5-10 mL or 1-2 teaspoons) of water. This suspension can be administered by spoon or by dropper.DO NOT STORE THE SUSPENSION.Foods that decrease absorption of levothyroxine, such as soybean infant formula, should not be used for administering levothyroxine sodium tablets (seePRECAUTIONS - Drug-Food Interactions).
10-15 mcg/kg/day. A lower starting dose (e.g., 25 mcg/day) should be considered in infants at risk for cardiac failure, and the dose should be increased in 4-6 weeks as needed based on clinical and laboratory response to treatment. In infants with very low (< 5 mcg/dL) or undetectable serum T4 concentrations, the recommended initial starting dose is50 mcg/dayof levothyroxine sodium.
Table 3). However, in children with chronic or severe hypothyroidism, an initial dose of25 mcg/dayof levothyroxine sodium is recommended with increments of 25 mcg every 2-4 weeks until the desired effect is achieved.
Table 3. Levothyroxine Sodium Dosing Guidelines for Pediatric Hypothyroidism
AGE Daily Dose Per Kg Body Weighta PRECAUTIONS - Laboratory TestsandPediatric Use).
PREGNANCY).
1 mcg/kg/day) than that used for full replacement may be adequate to normalize the serum TSH level. Patients who are not treated should be monitored yearly for changes in clinical status and thyroid laboratory parameters.
greater than 2 mcg/kg/day.However, in patients with high-risk tumors, the target level for TSH suppression may be < 0.01 mU/L.
CONTRAINDICATIONS,WARNINGSandPRECAUTIONS).
HOW SUPPLIED
SYNTHROID (levothyroxine sodium tablets, USP)are round, color coded, scored and debossed with "SYNTHROID" on one side and potency on the other side. They are supplied as follows:Strength (mcg) Color
NDC # for bottles of 100 NDC # for bottles of 1000 NDC # for unit dose cartons of 100
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


SynthroidLEVOTHYROXINE SODIUM TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!