SyDerm Complex
Syntrion SyDerm Complex
FULL PRESCRIBING INFORMATION: CONTENTS*
- SyDerm Complex Uses
- Warnings
- Directions
- SyDerm Complex Other information
- Inactive ingredients
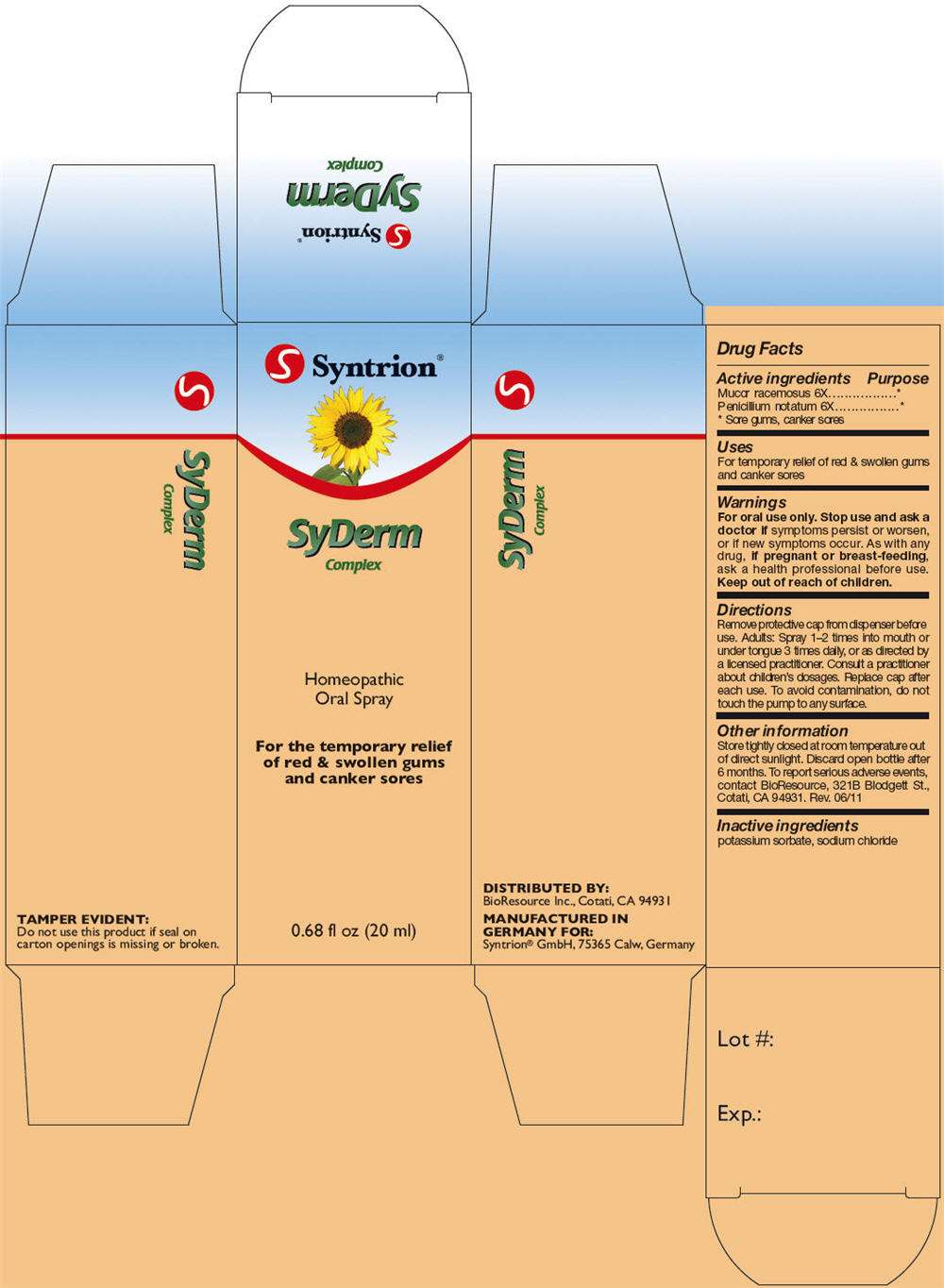
- PRINCIPAL DISPLAY PANEL - 20 ml Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Mucor racemosus 6X |
 |
| Penicillium notatum 6X |
 |
SyDerm Complex Uses
For temporary relief of red & swollen gums and canker sores
Warnings
For oral use only.
Stop use and ask a doctor if symptoms persist or worsen, or if new symptoms occur.
As with any drug, if pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Directions
Remove protective cap from dispenser before use. Adults: Spray 1–2 times into mouth or under tongue 3 times daily, or as directed by a licensed practitioner. Consult a practitioner about children's dosages. Replace cap after each use. To avoid contamination, do not touch the pump to any surface.
SyDerm Complex Other information
Store tightly closed at room temperature out of direct sunlight. Discard open bottle after 6 months. To report serious adverse events, contact BioResource, 321B Blodgett St., Cotati, CA 94931. Rev. 06/11
Inactive ingredients
potassium sorbate, sodium chloride
DISTRIBUTED BY:
BioResource Inc., Cotati, CA 94931
MANUFACTURED IN
GERMANY FOR:
Syntrion® GmbH, 75365 Calw, Germany
PRINCIPAL DISPLAY PANEL - 20 ml Bottle Carton
S Syntrion ®
SyDerm
Complex
Homeopathic
Oral Spray
For the temporary relief
of red & swollen gums
and canker sores
0.68 fl oz (20 ml)
SyDerm Complexmucor racemosus and penicillium chrysogenum var. chrysogenum SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||