Sunmark stay awake
McKesson Stay Awake Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Sunmark stay awake Uses
- Warnings
- Directions
- Sunmark stay awake Other information
- Inactive ingredients
- Questions or comments? 1-800-719-9260
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Caffeine 200 mg
Purpose
Alertness aid
Sunmark stay awake Uses
helps restore mental alertness or wakefulness when experiencing fatigue or drowsiness
Warnings
For occasional use only
Do not use
- in children under 12 years of age
- as a substitute for sleep
When using this product
limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as two cups of coffee.
Stop use and ask a doctor if
fatigue or drowsiness persists or continues to recur
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
adults and children 12 years of age and over: take 1 tablet not more often than every 3 to 4 hours
Sunmark stay awake Other information
- store at 20-25°C (68-77°F)
Inactive ingredients
colloidal silicon dioxide, D&C yellow #10 aluminum lake, dextrose, FD&C yellow #6 aluminum lake, magnesium stearate, maltodextrin, microcrystalline cellulose, pregelatinized starch
Questions or comments? 1-800-719-9260
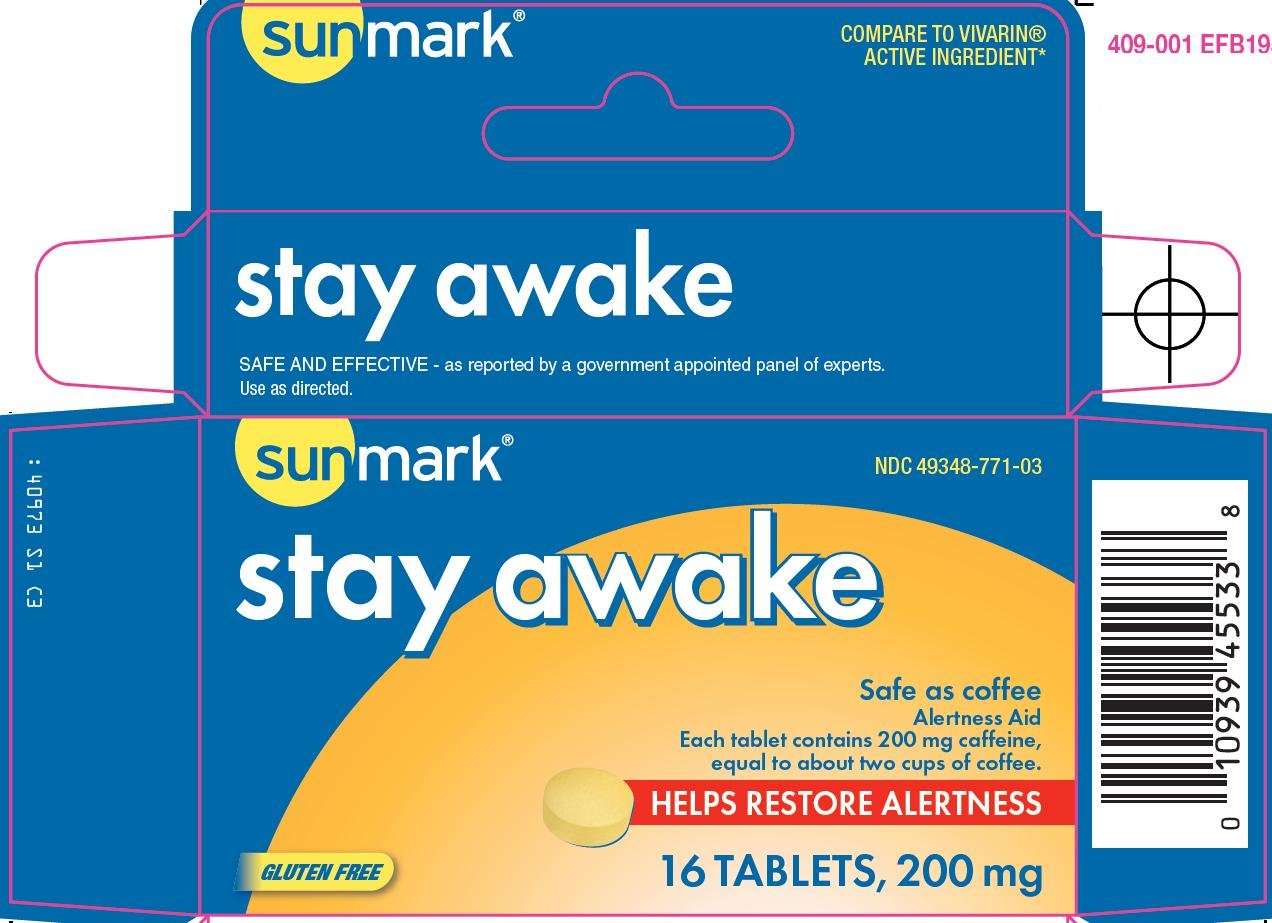
Principal Display Panel
COMPARE TO VIVARIN® ACTIVE INGREDIENT
stay awake
SAFE AND EFFECTIVE - as reported by a government appointed panel of experts.
Use as directed.
Safe as coffee
Alertness Aid
Each tablet contains 200 mg caffeine, equal to about two cups of coffee.
HELPS RESTORE ALERTNESS
GLUTEN FREE
200 mg
Sunmark stay awakeCaffeine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||